
Define conductivity and molar conductivity for the solution of an electrolyte. Discuss the variation of molar conductivity of weak electrolyte with dilution by giving a graph also
Answer
588.6k+ views
Hint: Conductance, Molar Conductance, Molar conductivity, increases with dilution as, on dilution a greater number of ions are produced, also they are much free to move or to conduct. For a weak electrolyte the variation of molar conductivity with dilution can be found by plotting the concentration against molar conductivity.
Complete step by step solution:
-Conductivity of an electrolyte is also called as specific conductance. It is the measure of ability of an electrolyte to conduct electricity.
- Conductivity is expressed in Siemens per meter in SI unit $\left( {S}/{m}\; \right)$.
-As we are aware, molar conductivity of a solution at a given concentration is the conductance of volume V of a solution containing one mole of electrolyte kept between two electrodes with an area of cross section A and distance of unit length. It can be represented as follows
${{\Lambda }_{m}}=\dfrac{K}{c}$
Where ${{\Lambda }_{m}}$ is the molar conductivity
$K$ is the specific conductivity
$c$ is the concentration in moles per volume.
Since as per the definition of molar conductivity, the solution contains only one mole of electrolyte and thus the above equation can be rewritten as
${{\Lambda }_{m}}=K V$
Where V is the total volume.
- Since conductivity is the conductance per centimeter cube of the solution, on dilution the concentration of ions per centimeter cube decreases and as a result conductivity also decreases.
-We are asked to find the variation of molar conductance of weak electrolyte on dilution. In the case of weak electrolytes, they dissociate into ions to a much lesser extent when compared to that of strong electrolyte.
- We have seen that dilution conductivity would decrease. But that is not the case with molar conductance. On dilution volume containing one mole of an electron increases and from the above equation, we could see that molar conductivity will increase with volume. Hence, molar conductivity increases with dilution.
- Debye Huckel Onsager equation gives us ideas about variation of molar conductivity of a weak electrolyte with dilution.
${{\Lambda }_{m}}={{\Lambda }^{0}}_{m}-A\sqrt{c}$
Where A is a constant and ${{\Lambda }^{0}}_{m}$ is the molar conductance at infinite dilution (limiting molar conductivity). The variation of ${{\Lambda }_{m}}$ with concentration can be studied by plotting ${{\Lambda }_{m}}$ against $\sqrt{c}$.For a weak electrolyte the plotted graph can be shown as
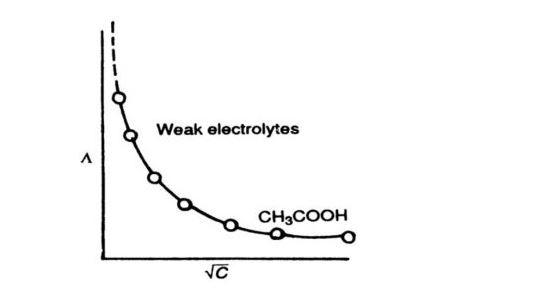
As we can observe from the graph, weak electrolytes have lower molar conductivities and lower degree of dissociation at higher concentrations which increases steeply at lower concentrations. As a result, limiting molar conductivity, ${{\Lambda }^{0}}_{m}$ cannot be obtained by extrapolation of molar conductivity to zero concentration. Hence, we have to use Kohlrausch law of independent migration of ions for determining to limiting molar conductivity, ${{\Lambda }^{0}}_{m}$ of weak electrolytes.
Note: The terminologies must be used cautiously. Conductance is the reverse of electrical resistance; conductivity is the inverse of resistivity. Molar conductance is conductance of all the ions produced by ionization of 1 g mole of an electrolyte which is present in V mL of solution. Molar conductivity is conductivity of one mole of electrolyte.
Complete step by step solution:
-Conductivity of an electrolyte is also called as specific conductance. It is the measure of ability of an electrolyte to conduct electricity.
- Conductivity is expressed in Siemens per meter in SI unit $\left( {S}/{m}\; \right)$.
-As we are aware, molar conductivity of a solution at a given concentration is the conductance of volume V of a solution containing one mole of electrolyte kept between two electrodes with an area of cross section A and distance of unit length. It can be represented as follows
${{\Lambda }_{m}}=\dfrac{K}{c}$
Where ${{\Lambda }_{m}}$ is the molar conductivity
$K$ is the specific conductivity
$c$ is the concentration in moles per volume.
Since as per the definition of molar conductivity, the solution contains only one mole of electrolyte and thus the above equation can be rewritten as
${{\Lambda }_{m}}=K V$
Where V is the total volume.
- Since conductivity is the conductance per centimeter cube of the solution, on dilution the concentration of ions per centimeter cube decreases and as a result conductivity also decreases.
-We are asked to find the variation of molar conductance of weak electrolyte on dilution. In the case of weak electrolytes, they dissociate into ions to a much lesser extent when compared to that of strong electrolyte.
- We have seen that dilution conductivity would decrease. But that is not the case with molar conductance. On dilution volume containing one mole of an electron increases and from the above equation, we could see that molar conductivity will increase with volume. Hence, molar conductivity increases with dilution.
- Debye Huckel Onsager equation gives us ideas about variation of molar conductivity of a weak electrolyte with dilution.
${{\Lambda }_{m}}={{\Lambda }^{0}}_{m}-A\sqrt{c}$
Where A is a constant and ${{\Lambda }^{0}}_{m}$ is the molar conductance at infinite dilution (limiting molar conductivity). The variation of ${{\Lambda }_{m}}$ with concentration can be studied by plotting ${{\Lambda }_{m}}$ against $\sqrt{c}$.For a weak electrolyte the plotted graph can be shown as

As we can observe from the graph, weak electrolytes have lower molar conductivities and lower degree of dissociation at higher concentrations which increases steeply at lower concentrations. As a result, limiting molar conductivity, ${{\Lambda }^{0}}_{m}$ cannot be obtained by extrapolation of molar conductivity to zero concentration. Hence, we have to use Kohlrausch law of independent migration of ions for determining to limiting molar conductivity, ${{\Lambda }^{0}}_{m}$ of weak electrolytes.
Note: The terminologies must be used cautiously. Conductance is the reverse of electrical resistance; conductivity is the inverse of resistivity. Molar conductance is conductance of all the ions produced by ionization of 1 g mole of an electrolyte which is present in V mL of solution. Molar conductivity is conductivity of one mole of electrolyte.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




