
Define octet rule and write its significance and limitations.
Answer
598.2k+ views
Hint – You can start the solution by describing how the rule of octet came in existence, then move on to defining the octet rule. Then you can write down its significance in explaining the formation and stability of compounds. Follow it up by defining what the limitations of this rule are with specific examples to better explain it.
Complete step by step solution:
> In scientific history we have seen multiple attempts of solving the mystery of how atoms bind with each other. In 1904, we saw a significant move forward when Richard Abegg proposed the concept of coordination number. This concept is considered as an extension to the concept of valency (the combining power of an element). He proposed that atoms can behave as either donor or acceptors of electrons. The octet rule uses this idea as a basis and states that every atom binds with another atom in order to have eight electrons in its outermost shell by donating or accepting electrons.
> Significance Of Octet Rule – This rule is able successfully explain the formation of various compounds and explain the stability of the compound. Example – Carbon dioxide (\[C{O_2}\])
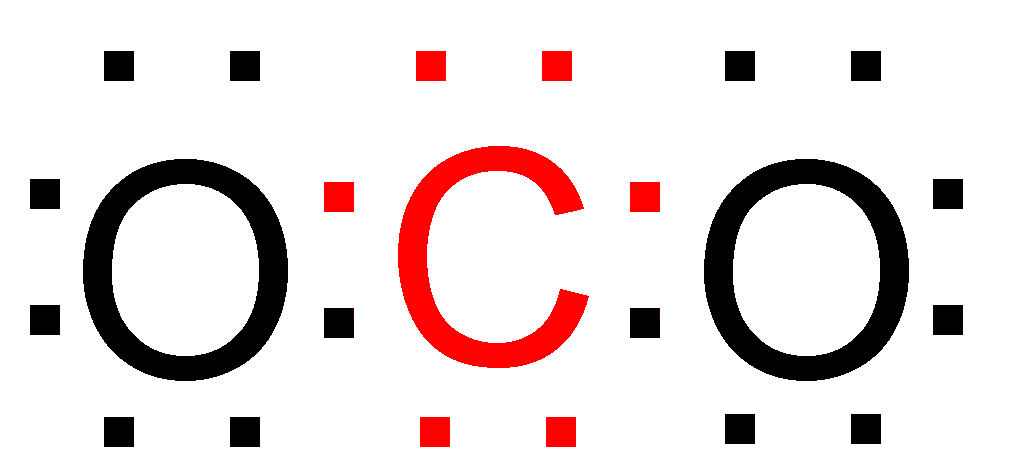
As you can see both the \[C\] atom the two \[O\] atoms after bonding have eight electrons in their outermost shell, and as predicted by the octet rule \[C{O_2}\] is relatively stable in nature.
Limitations –
Hydrogen and helium atoms have only one electron shell and hence it has only \[1{s^2}\] shell. So it is usually impossible for hydrogen and helium to have eight electrons in its outermost shell.
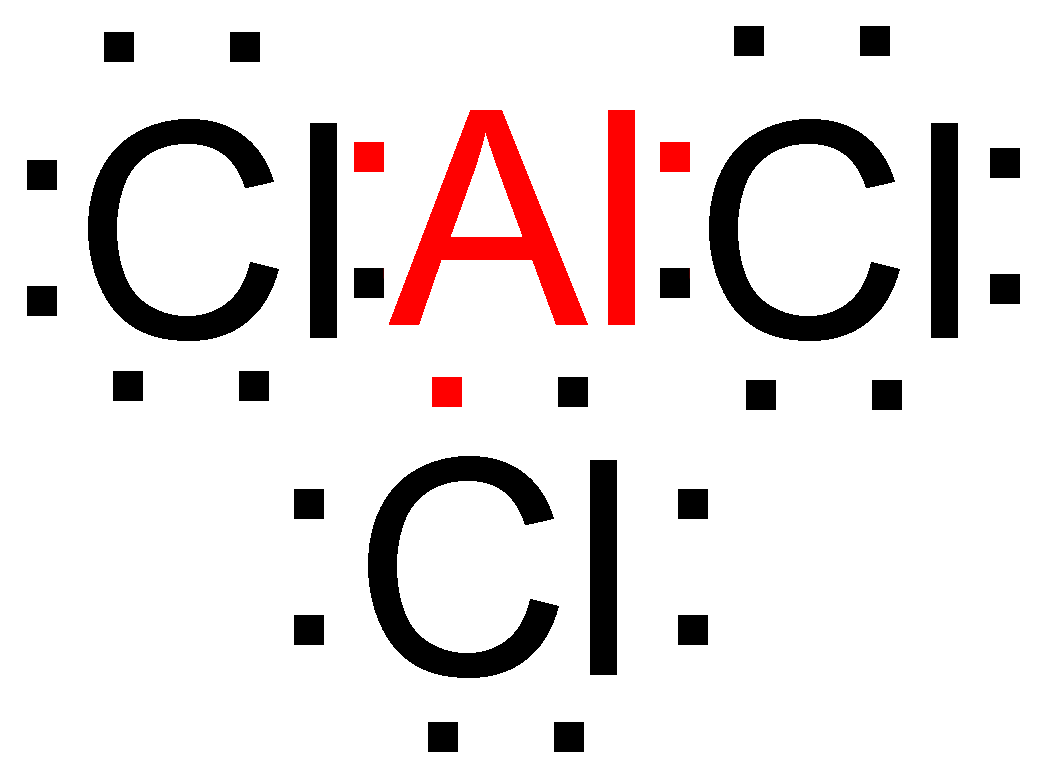
> We have compounds in which the atoms have less than 8 electrons in their outermost shell but are still relatively stable in nature. Example – In Aluminum chloride (\[AlC{l_3}\]), the aluminum only has 6 electrons in its outermost shell even after bonding.
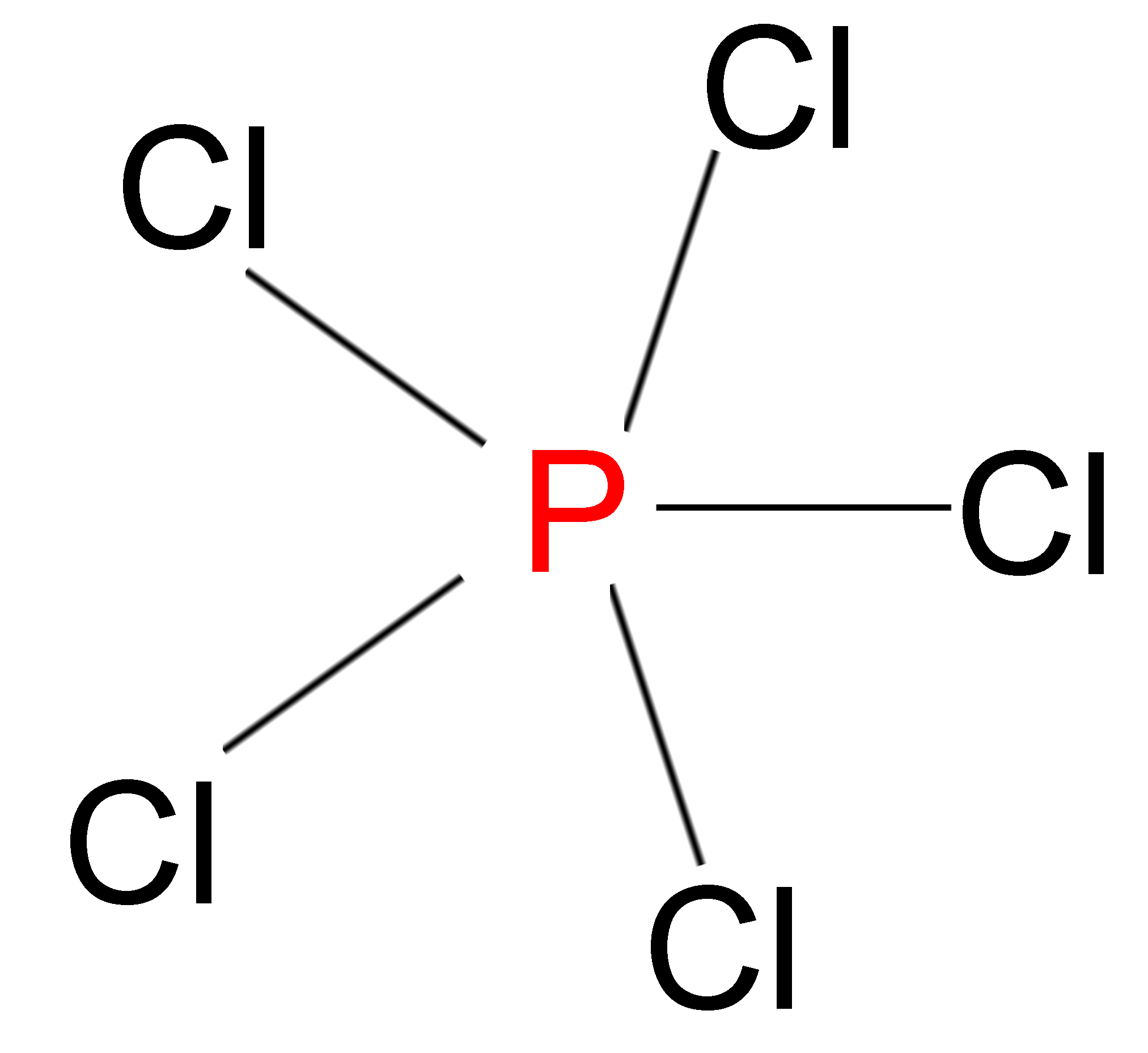
> We also have compounds in which the atoms have more than 8 electrons in their outermost shell but are still stable in nature. Example – In phosphorus pentachloride (\[PC{l_5}\]), the Phosphorus atom has 10 electrons in its outermost shell.
This rule cannot be used to predict the shape and stability of molecules.
> This rule is based on the concept that the electronic configuration of inert gases (have 8 electrons in the outermost shell) are the most stable and every atom bonds in order to achieve similar configuration. But inert gases like xenon (\[Xe\]) and krypton (\[Kr\]) form compounds like\[Xe{F_2}\], \[Xe{F_6}\], \[Kr{F_2}\] etc.
Note – In the solution given above, we have seen multiple examples of compounds that follow or do not follow octet rule. A common theme in all those compounds was that all of the atoms there after bonding had even numbers of electrons surrounding them. But we also have compounds in which some atoms have an odd number of electrons in their outermost shell. For example- In $[NO]$, the $N$ atom has 7 electrons in the outermost shells.
Complete step by step solution:
> In scientific history we have seen multiple attempts of solving the mystery of how atoms bind with each other. In 1904, we saw a significant move forward when Richard Abegg proposed the concept of coordination number. This concept is considered as an extension to the concept of valency (the combining power of an element). He proposed that atoms can behave as either donor or acceptors of electrons. The octet rule uses this idea as a basis and states that every atom binds with another atom in order to have eight electrons in its outermost shell by donating or accepting electrons.
> Significance Of Octet Rule – This rule is able successfully explain the formation of various compounds and explain the stability of the compound. Example – Carbon dioxide (\[C{O_2}\])

As you can see both the \[C\] atom the two \[O\] atoms after bonding have eight electrons in their outermost shell, and as predicted by the octet rule \[C{O_2}\] is relatively stable in nature.
Limitations –
Hydrogen and helium atoms have only one electron shell and hence it has only \[1{s^2}\] shell. So it is usually impossible for hydrogen and helium to have eight electrons in its outermost shell.
> We have compounds in which the atoms have less than 8 electrons in their outermost shell but are still relatively stable in nature. Example – In Aluminum chloride (\[AlC{l_3}\]), the aluminum only has 6 electrons in its outermost shell even after bonding.

> We also have compounds in which the atoms have more than 8 electrons in their outermost shell but are still stable in nature. Example – In phosphorus pentachloride (\[PC{l_5}\]), the Phosphorus atom has 10 electrons in its outermost shell.

This rule cannot be used to predict the shape and stability of molecules.
> This rule is based on the concept that the electronic configuration of inert gases (have 8 electrons in the outermost shell) are the most stable and every atom bonds in order to achieve similar configuration. But inert gases like xenon (\[Xe\]) and krypton (\[Kr\]) form compounds like\[Xe{F_2}\], \[Xe{F_6}\], \[Kr{F_2}\] etc.
Note – In the solution given above, we have seen multiple examples of compounds that follow or do not follow octet rule. A common theme in all those compounds was that all of the atoms there after bonding had even numbers of electrons surrounding them. But we also have compounds in which some atoms have an odd number of electrons in their outermost shell. For example- In $[NO]$, the $N$ atom has 7 electrons in the outermost shells.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




