
Dehydration of alcohol is an example of which type of reaction?
(A) Substitution
(B) Elimination
(C) Addition
(D) Both B and C
Answer
586.5k+ views
Hint: During dehydration, a protic acid reacts with alcohol, a carbocation is formed by losing a water molecule and then again the loss of a proton from neighbouring carbon occurs leading to the formation of the alkene. So, this reaction mainly involves loss of water molecules and the formation of the alkene.
Complete step by step solution:
-First, we will see what dehydration of alcohol is.
Dehydration of alcohol means losing a water molecule. When a molecule of the alcohol reacts with some protic acid, it loses a water molecule and leads to the formation of alkenes. These reactions are known as dehydration of alcohols and are an important example of elimination reactions.
-Now we will see how this reaction of dehydration of alcohol takes place. The dehydration of alcohol will follow either the E1 or E2 mechanism. The primary alcohols will undergo elimination reaction via the E1 mechanism, while the secondary and tertiary alcohols undergo elimination via the E2 mechanism.
For example: $C{H_3} - C{H_2}OH\xrightarrow{{alc.KOH}}C{H_2} = C{H_2}$
Its mechanism is as follows: (we will see this with the help of the mechanism of the above-given example)
(1)Formation of a protonated alcohol: In this step, the protic acid (like alc. KOH, ${H_2}S{O_4}$) attacks on the alcohol and there is the attachment of a proton on the alcoholic oxygen atom. This occurs because there is a single pair of electrons present on the oxygen atom which makes it behave like a Lewis base. This step is reversible.
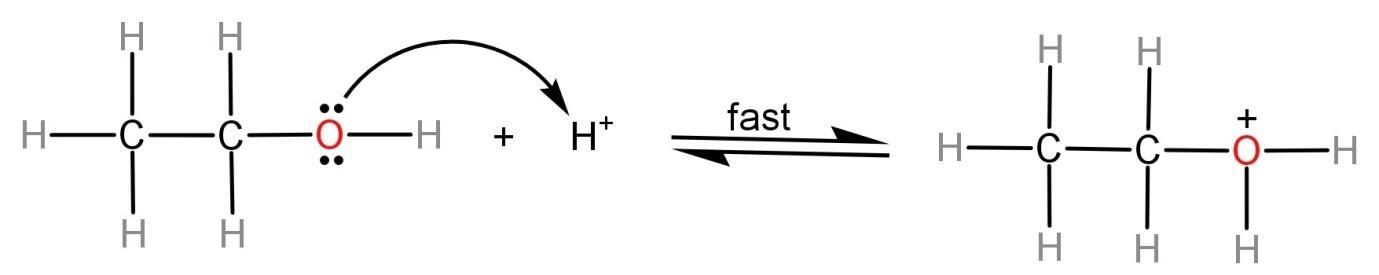
(2)Formation of a carbocation: This is the slowest step and hence the rate-determining step. In this step the bond between the carbon and oxygen breaks (C-O) leading to the formation of a carbocation.
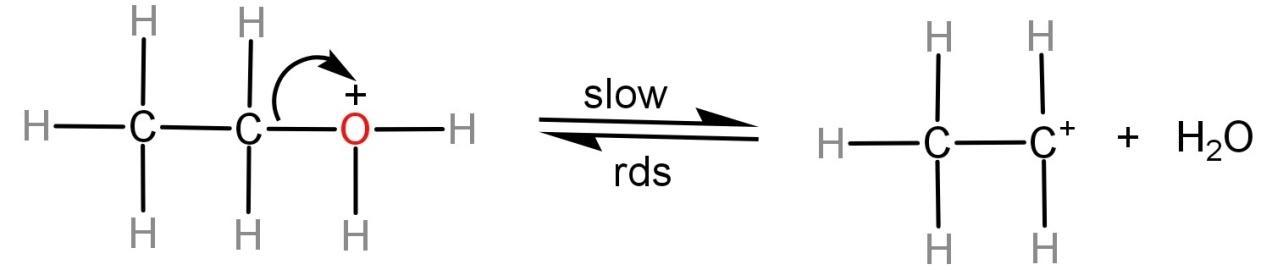
(3)Formation of alkenes: It is the final step. In this step the proton earlier generated is eliminated using a base and the carbon atom neighbouring to the carbocation breaks its C-H bond, leading to the formation of an alkene (C=C).
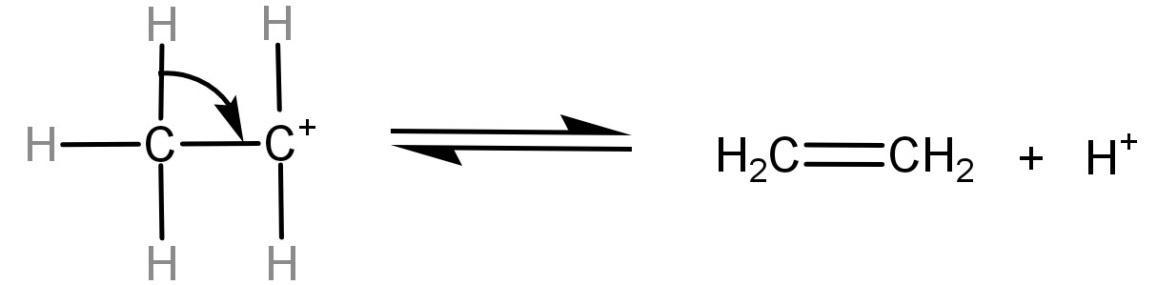
Hence the correct option is: (B) Elimination
Note: Since the mechanism involves the formation of the carbocation, the rates of dehydration of alcohols will differ in primary, secondary and tertiary alcohols. Tertiary carbocations are most stable and hence tertiary alcohols have the highest rate of dehydration than secondary and primary alcohols.
Rate of dehydration of alcohols: Tertiary > Secondary > Primary > Methanol
Complete step by step solution:
-First, we will see what dehydration of alcohol is.
Dehydration of alcohol means losing a water molecule. When a molecule of the alcohol reacts with some protic acid, it loses a water molecule and leads to the formation of alkenes. These reactions are known as dehydration of alcohols and are an important example of elimination reactions.
-Now we will see how this reaction of dehydration of alcohol takes place. The dehydration of alcohol will follow either the E1 or E2 mechanism. The primary alcohols will undergo elimination reaction via the E1 mechanism, while the secondary and tertiary alcohols undergo elimination via the E2 mechanism.
For example: $C{H_3} - C{H_2}OH\xrightarrow{{alc.KOH}}C{H_2} = C{H_2}$
Its mechanism is as follows: (we will see this with the help of the mechanism of the above-given example)
(1)Formation of a protonated alcohol: In this step, the protic acid (like alc. KOH, ${H_2}S{O_4}$) attacks on the alcohol and there is the attachment of a proton on the alcoholic oxygen atom. This occurs because there is a single pair of electrons present on the oxygen atom which makes it behave like a Lewis base. This step is reversible.

(2)Formation of a carbocation: This is the slowest step and hence the rate-determining step. In this step the bond between the carbon and oxygen breaks (C-O) leading to the formation of a carbocation.

(3)Formation of alkenes: It is the final step. In this step the proton earlier generated is eliminated using a base and the carbon atom neighbouring to the carbocation breaks its C-H bond, leading to the formation of an alkene (C=C).

Hence the correct option is: (B) Elimination
Note: Since the mechanism involves the formation of the carbocation, the rates of dehydration of alcohols will differ in primary, secondary and tertiary alcohols. Tertiary carbocations are most stable and hence tertiary alcohols have the highest rate of dehydration than secondary and primary alcohols.
Rate of dehydration of alcohols: Tertiary > Secondary > Primary > Methanol
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




