
Dehydration of alcohols take place more rapidly with $POC{{l}_{3}}$ than with ${{H}_{2}}S{{O}_{4}}$.
Select the correct statement(s) about the above dehydration reaction:
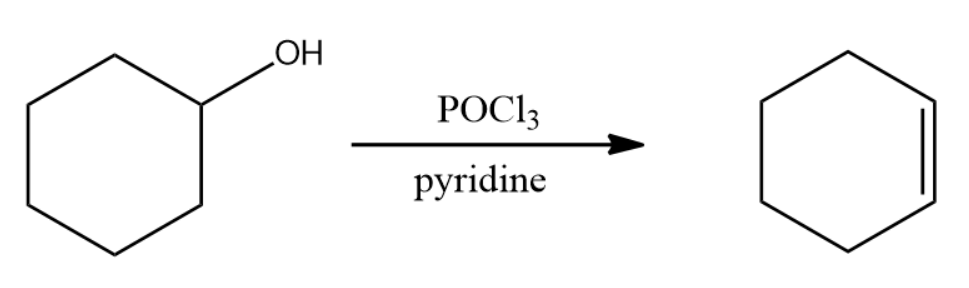
This question has multiple correct options

Answer
560.7k+ views
Hint: An attempt to this question can be made by determining the reaction mechanism for dehydration using both the reagents $POC{{l}_{3}}$ and${{H}_{2}}S{{O}_{4}}$. Now determine the difference in the mechanism. With this you can reason why $POC{{l}_{3}}$ is preferred over ${{H}_{2}}S{{O}_{4}}$ for dehydration.
Complete step by step answer:
So in the question it is asked that, we have to select
We will write the reaction mechanism for dehydration using $POC{{l}_{3}}$.
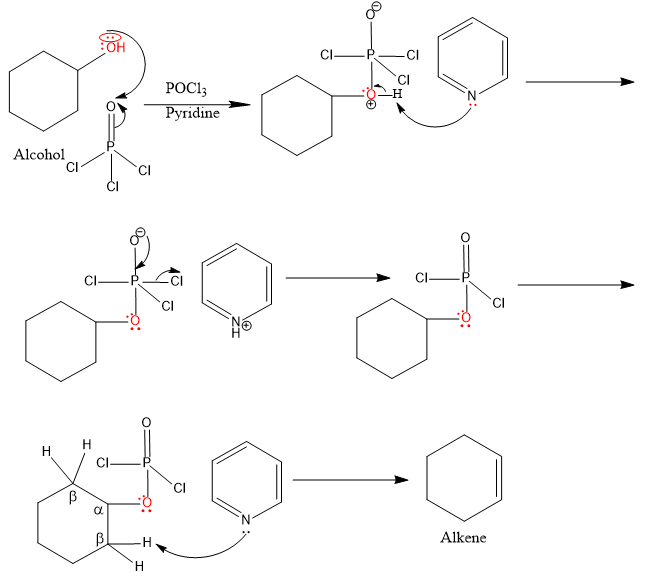
In the above reaction we observe,
- No carbocation formation
- Nucleophilic attack used for deprotonation
- involves E2 mechanism as pyridine base abstracts proton from the adjacent carbon as the same time at which $-\text{OPOC}{{\text{l}}_{2}}$ is leaving
- involves $\text{R}-\text{OPOC}{{\text{l}}_{2}}$ with $-\text{OPOC}{{\text{l}}_{2}}$ as a better leaving group
Based on the above statements we can conclude that the below given options stand correct.
(A) It does not involve carbocation.
(B) It involves the species $\text{R}-\text{OPOC}{{\text{l}}_{2}}$ with $-\text{OPOC}{{\text{l}}_{2}}$ as a better leaving group.
(C) It involves E2 mechanism as the pyridine base abstracts proton from the adjacent carbon as the same time at which $-\text{OPOC}{{\text{l}}_{2}}$ is leaving.
The correct answer is option “A, B and C” .
Note: The reaction with sulfuric acid on the other hand involves the formation of carbocation. This is a slow step. Along with that it uses the E1 mechanism for elimination unlike $POC{{l}_{3}}$ that uses the E2 mechanism for elimination.
Complete step by step answer:
So in the question it is asked that, we have to select
We will write the reaction mechanism for dehydration using $POC{{l}_{3}}$.

In the above reaction we observe,
- No carbocation formation
- Nucleophilic attack used for deprotonation
- involves E2 mechanism as pyridine base abstracts proton from the adjacent carbon as the same time at which $-\text{OPOC}{{\text{l}}_{2}}$ is leaving
- involves $\text{R}-\text{OPOC}{{\text{l}}_{2}}$ with $-\text{OPOC}{{\text{l}}_{2}}$ as a better leaving group
Based on the above statements we can conclude that the below given options stand correct.
(A) It does not involve carbocation.
(B) It involves the species $\text{R}-\text{OPOC}{{\text{l}}_{2}}$ with $-\text{OPOC}{{\text{l}}_{2}}$ as a better leaving group.
(C) It involves E2 mechanism as the pyridine base abstracts proton from the adjacent carbon as the same time at which $-\text{OPOC}{{\text{l}}_{2}}$ is leaving.
The correct answer is option “A, B and C” .
Note: The reaction with sulfuric acid on the other hand involves the formation of carbocation. This is a slow step. Along with that it uses the E1 mechanism for elimination unlike $POC{{l}_{3}}$ that uses the E2 mechanism for elimination.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




