
Describe a method of refining nickel.
Answer
590.4k+ views
Hint: Nickel easily forms a volatile compound with available reagent. This volatile compound can then be readily decomposed to facilitate the recovery of metal. Nickel is refined by mond’s process.
Complete answer:
Some of the methods that can be used for the refining of metals are distillation, liquation, poling, electrolysis, zone refining and vapour phase refining.
Impure nickel metal is obtained from the reaction of nickel oxide with syngas at 473 K. Nickel metal thus formed has some impurities of iron and cobalt.
${NiO(s) + {H_2}(g) \to {Ni}(s) + {H_2}O}$
\[{\rm{NiO(s) + }}{{\rm{H}}_{\rm{2}}}{\rm{(g) }} \to {\rm{ Ni(s) + }}{{\rm{H}}_{\rm{2}}}{\rm{O(g)}}{\rm{.}}\]
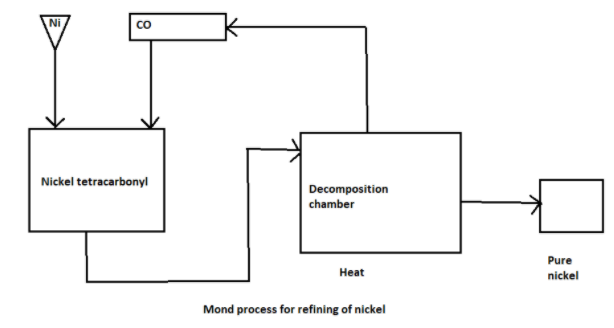
Nickel is refined by mond’s process. Impure nickel reacts with carbon monoxide at around
330-350 K to form nickel tetracarbonyl. Nickel tetracarbonyl is a volatile compound and is obtained as a vapour. The impurities are left behind.
At a high temperature of around 450-470 K, nickel tetracarbonyl is decomposed to regenerate pure nickel.
Write the balanced chemical equations for the process.
$\text{Ni + 4 CO }\to \text{ Ni}{{\left( \text{CO} \right)}_{4}}$
$\text{Ni}{{\left( \text{CO} \right)}_{4}}\to \text{ Ni + 4 CO}$
In the first reaction,one nickel atom combines with four carbon monoxide molecules to form nickel tetracarbonyl. In the next step, one molecule of nickel tetrachloride decomposes to give one nickel atom and four carbon monoxide ligands.
The diagram for the Mond process is as follows:
Note: In the vapour phase refining technique, metal to be purified, is converted into a volatile compound by reaction with suitable reagent. In the next step, this volatile compound of metal is decomposed to obtain pure metal. The vapour phase refining technique is used for the refining of nickel, titanium and zirconium metals.
Complete answer:
Some of the methods that can be used for the refining of metals are distillation, liquation, poling, electrolysis, zone refining and vapour phase refining.
Impure nickel metal is obtained from the reaction of nickel oxide with syngas at 473 K. Nickel metal thus formed has some impurities of iron and cobalt.
${NiO(s) + {H_2}(g) \to {Ni}(s) + {H_2}O}$
\[{\rm{NiO(s) + }}{{\rm{H}}_{\rm{2}}}{\rm{(g) }} \to {\rm{ Ni(s) + }}{{\rm{H}}_{\rm{2}}}{\rm{O(g)}}{\rm{.}}\]
Nickel is refined by mond’s process. Impure nickel reacts with carbon monoxide at around
330-350 K to form nickel tetracarbonyl. Nickel tetracarbonyl is a volatile compound and is obtained as a vapour. The impurities are left behind.
At a high temperature of around 450-470 K, nickel tetracarbonyl is decomposed to regenerate pure nickel.
Write the balanced chemical equations for the process.
$\text{Ni + 4 CO }\to \text{ Ni}{{\left( \text{CO} \right)}_{4}}$
$\text{Ni}{{\left( \text{CO} \right)}_{4}}\to \text{ Ni + 4 CO}$
In the first reaction,one nickel atom combines with four carbon monoxide molecules to form nickel tetracarbonyl. In the next step, one molecule of nickel tetrachloride decomposes to give one nickel atom and four carbon monoxide ligands.
The diagram for the Mond process is as follows:

Note: In the vapour phase refining technique, metal to be purified, is converted into a volatile compound by reaction with suitable reagent. In the next step, this volatile compound of metal is decomposed to obtain pure metal. The vapour phase refining technique is used for the refining of nickel, titanium and zirconium metals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




