
Describe an experiment to verify Boyle's law.
Answer
588.6k+ views
Hint:
Boyle’s law is a law stating that the pressure of a given mass of an ideal gas is inversely proportional to its volume at a constant temperature.
Complete step-by-step answer:
Objective:
To verify Boyle's law i.e. for a given amount of gas, absolute pressure is inversely proportional to volume at a constant temperature.
Applications:
Air column with measuring scale, Bourdon pressure gauge, oil reservoir, hand air pump, barometer
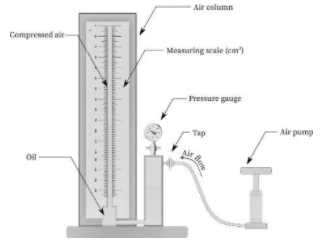
Procedure:
Connect the apparatus as shown in the above diagram. Then the connection of the oil reservoir to the air column must be such that there is no leakage and the air is completely sealed off by the oil. Now,the hand air pump is attached to the oil reservoir. After assembling all the types of equipment, open the air tap and start pumping air through the air pump. Keep pumping the air until the oil is reached in the upper part of the column. As the air flows in the oil reservoir, the pressure inside the system increases. This can be observed in the pressure gauge. Now, close the air tap once the oil no longer rises and the pressure gauge reading is constant (i.e. reached its peak). Wait for two to three minutes to cool down the compressed air and note the pressure reading and the volume reading. Now, slightly open and then quickly closed the air tap to let some air escape the system. This will lower the oil in the column. After two to three minutes, note the pressure and volume reading. Repeat the above two steps such that seven to eight observations are recorded. Prepare the observation table and follow the calculations as shown below. Plot pressure vs volume and pressure vs inverse volume graph. Finally, conclude the experiment based on the graphs.
Note:
Make sure that the air of the column should be completely sealed off by the oil. And every reading must be taken for two or three of closing the air tap.
Boyle’s law is a law stating that the pressure of a given mass of an ideal gas is inversely proportional to its volume at a constant temperature.
Complete step-by-step answer:
Objective:
To verify Boyle's law i.e. for a given amount of gas, absolute pressure is inversely proportional to volume at a constant temperature.
Applications:
Air column with measuring scale, Bourdon pressure gauge, oil reservoir, hand air pump, barometer

Procedure:
Connect the apparatus as shown in the above diagram. Then the connection of the oil reservoir to the air column must be such that there is no leakage and the air is completely sealed off by the oil. Now,the hand air pump is attached to the oil reservoir. After assembling all the types of equipment, open the air tap and start pumping air through the air pump. Keep pumping the air until the oil is reached in the upper part of the column. As the air flows in the oil reservoir, the pressure inside the system increases. This can be observed in the pressure gauge. Now, close the air tap once the oil no longer rises and the pressure gauge reading is constant (i.e. reached its peak). Wait for two to three minutes to cool down the compressed air and note the pressure reading and the volume reading. Now, slightly open and then quickly closed the air tap to let some air escape the system. This will lower the oil in the column. After two to three minutes, note the pressure and volume reading. Repeat the above two steps such that seven to eight observations are recorded. Prepare the observation table and follow the calculations as shown below. Plot pressure vs volume and pressure vs inverse volume graph. Finally, conclude the experiment based on the graphs.
Note:
Make sure that the air of the column should be completely sealed off by the oil. And every reading must be taken for two or three of closing the air tap.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Which Country is Called "The Land of Festivals"?

What type of cell is found in the Seminiferous tub class 10 biology CBSE

What are the public facilities provided by the government? Also explain each facility




