
Describe the method for the purification of colloidal solution containing excess amount of electrolytes with diagram:
Answer
600.6k+ views
Hint: Generally, freshly prepared colloidal solutions usually contain the impurities of electrolytes. The presence of electrolytes in large concentrations tends to destabilize the colloidal solution.
Complete step by step answer:
Let us first understand about dialysis process,
(1) Dialysis:
Dialysis is the process which is used for the separation of crystalloids from a colloid by filtration or diffusion through parchment paper or animal membrane.
The apparatus employed for this purpose is called a dialyser. . .
It consists of a bag made of cellophane (membrane).
The bag is filled with the impure solution which has to be purified and is suspended in a tank through which pure water is circulated.
The impurities of electrolytes present in the solution diffuse out of the bag leaving pure solution in the bag.
(2) Electrodialysis:
As we know dialysis is a slow process. Electrodialysis is a process in which electrically charged membranes are used to separate ions from an aqueous solution by driving force of an electrical potential difference. Electrodialysis is used in combination with bipolar membranes for the production of acids and bases from waste water containing salts.
Let us understand better with the help of a diagram.
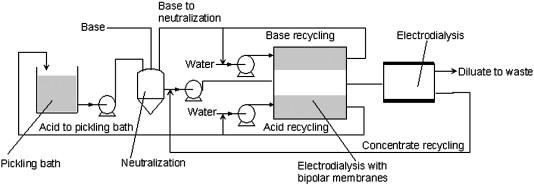
Now if we consider the question, Electrodialysis is the method for the purification of a colloidal solution containing excess amounts of electrolytes.
Note: Electrodialysis is used today to perform several general types of separations, like the separation of ionic compounds from neutral molecules, separation of monovalent ions from multivalent ions etc.
Complete step by step answer:
Let us first understand about dialysis process,
(1) Dialysis:
Dialysis is the process which is used for the separation of crystalloids from a colloid by filtration or diffusion through parchment paper or animal membrane.
The apparatus employed for this purpose is called a dialyser. . .
It consists of a bag made of cellophane (membrane).
The bag is filled with the impure solution which has to be purified and is suspended in a tank through which pure water is circulated.
The impurities of electrolytes present in the solution diffuse out of the bag leaving pure solution in the bag.
(2) Electrodialysis:
As we know dialysis is a slow process. Electrodialysis is a process in which electrically charged membranes are used to separate ions from an aqueous solution by driving force of an electrical potential difference. Electrodialysis is used in combination with bipolar membranes for the production of acids and bases from waste water containing salts.
Let us understand better with the help of a diagram.

Now if we consider the question, Electrodialysis is the method for the purification of a colloidal solution containing excess amounts of electrolytes.
Note: Electrodialysis is used today to perform several general types of separations, like the separation of ionic compounds from neutral molecules, separation of monovalent ions from multivalent ions etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




