
Describe with suitable diagrams the deacon process of manufacture of chlorine. Write its reaction with ammonia.
Answer
568.5k+ views
Hint:. Try to recall that chlorine is greenish yellow gas and has a strong pungent and suffocating smell odour. Also, due to its high electronegativity, chlorine is a very reactive element. Now, by using this you can easily answer the given question.
Complete step by step answer:
- It is known to you that there are a lot of methods by which chlorine gas can be prepared of which we will discuss about the deacon process.
In this process, hydrochloric acid is oxidized by atmospheric air in the presence of $CuC{l_2}$ (catalyst) at 723 K. The reaction is:
$HCl + {O_2}\xrightarrow[{Catalyst~723K}]{{CuC{l_2}}}2C{l_2} + 2{H_2}O$
- The above reaction is reversible and results in about 65% conversion. Nowadays, this method is modified by using improved catalyst $CuC{l_2}$ with didymium oxide as promoter, didymium is an old name meaning twin and it consists of two lanthanide elements praseodymium and neodymium). This process generally takes place at lower temperature.
- Now, we will see the action of ammonia with chlorine: The reaction of chlorine with ammonia depends upon the proportion of the reactants as:
If ammonia is in excess, nitrogen is formed. The reaction is as follows:
$8N{H_3} + 3C{l_2} \to 6N{H_4}Cl + {N_2}$
(excess)
- If chlorine is in excess, then nitrogen trichloride is formed. The reaction is as follows: $N{H_3} + 3C{l_2} \to NC{l_3} + HCl$ (Chlorine is in excess)
The diagram for deacon process is given below:
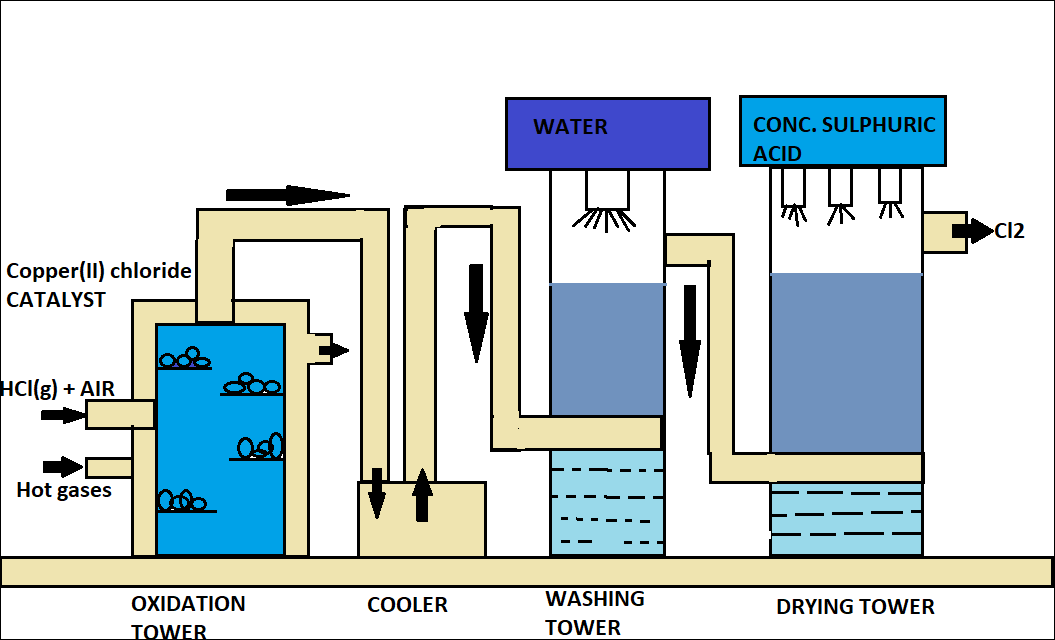
Note: It should be remembered to you that dry chlorine has no effect on litmus paper but in presence of moisture chlorine turns blue litmus red.
Also, you should remember that chlorine almost does not combine with hydrogen in the dark. However, in the presence of sunlight, chlorine combines with hydrogen explosively.
Complete step by step answer:
- It is known to you that there are a lot of methods by which chlorine gas can be prepared of which we will discuss about the deacon process.
In this process, hydrochloric acid is oxidized by atmospheric air in the presence of $CuC{l_2}$ (catalyst) at 723 K. The reaction is:
$HCl + {O_2}\xrightarrow[{Catalyst~723K}]{{CuC{l_2}}}2C{l_2} + 2{H_2}O$
- The above reaction is reversible and results in about 65% conversion. Nowadays, this method is modified by using improved catalyst $CuC{l_2}$ with didymium oxide as promoter, didymium is an old name meaning twin and it consists of two lanthanide elements praseodymium and neodymium). This process generally takes place at lower temperature.
- Now, we will see the action of ammonia with chlorine: The reaction of chlorine with ammonia depends upon the proportion of the reactants as:
If ammonia is in excess, nitrogen is formed. The reaction is as follows:
$8N{H_3} + 3C{l_2} \to 6N{H_4}Cl + {N_2}$
(excess)
- If chlorine is in excess, then nitrogen trichloride is formed. The reaction is as follows: $N{H_3} + 3C{l_2} \to NC{l_3} + HCl$ (Chlorine is in excess)
The diagram for deacon process is given below:

Note: It should be remembered to you that dry chlorine has no effect on litmus paper but in presence of moisture chlorine turns blue litmus red.
Also, you should remember that chlorine almost does not combine with hydrogen in the dark. However, in the presence of sunlight, chlorine combines with hydrogen explosively.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




