
Why is detergent better than soap in cleansing action?
Answer
580.5k+ views
Hint: Detergents are sodium salts of long chain benzene sulphonic acids. The ionic group in detergents is ${ SO }_{ 3 }^{ - }{ Na }^{ + } $ or $ { SO }_{ 4 }^{ - }{ Na }^{ + }$ while soaps are sodium and potassium salts of long chain fatty acids containing ${ 12 }$ to ${ 18 }$ carbon atoms.
Complete step-by-step answer:
The cleansing action of detergents:
Detergent when dissolved in water form micelle. These micelles help in the cleansing action of detergent. The cleansing action of detergent is very much similar to that of soap. The only difference is that detergents work with hard water also. They don’t form insoluble salts with calcium, magnesium, and iron ions as soaps. It does not react with Ca and Mg salts and hence do not form scum and form a large amount of lather.
It simply means detergents are better as cleaning agents as they work just as effectively in hard water as they do in soft water. Soap doesn't work properly in hard water (it forms scum with the calcium and magnesium impurities) but for detergents, there is no such problem.
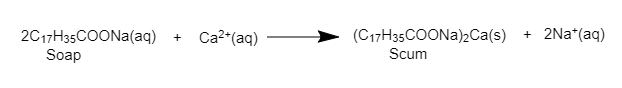
Hence, we can say that detergents are better than soaps in cleaning action.
Additional Information:
Detergent is a water-polluting agent as it is non-biodegradable and hence, does not decompose, hence posing a curse on the earth.
Uses of detergents:
1. It is effective in cleaning woolen clothes.
2. It is also used in many shampoos.
Note: The possibility to make a mistake is that you may confuse between soaps and detergents as soaps are sodium or potassium salts of fatty acids while detergents are sodium salts of benzene sulphonic acids.
Complete step-by-step answer:
The cleansing action of detergents:
Detergent when dissolved in water form micelle. These micelles help in the cleansing action of detergent. The cleansing action of detergent is very much similar to that of soap. The only difference is that detergents work with hard water also. They don’t form insoluble salts with calcium, magnesium, and iron ions as soaps. It does not react with Ca and Mg salts and hence do not form scum and form a large amount of lather.
It simply means detergents are better as cleaning agents as they work just as effectively in hard water as they do in soft water. Soap doesn't work properly in hard water (it forms scum with the calcium and magnesium impurities) but for detergents, there is no such problem.

Hence, we can say that detergents are better than soaps in cleaning action.
Additional Information:
Detergent is a water-polluting agent as it is non-biodegradable and hence, does not decompose, hence posing a curse on the earth.
Uses of detergents:
1. It is effective in cleaning woolen clothes.
2. It is also used in many shampoos.
Note: The possibility to make a mistake is that you may confuse between soaps and detergents as soaps are sodium or potassium salts of fatty acids while detergents are sodium salts of benzene sulphonic acids.
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

How much time does it take to bleed after eating p class 12 biology CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




