
How to determine the stability of gauche and anti conformation?
Answer
555.3k+ views
Hint: Conformational isomers are interconverted by rotations around a single bond. They are classified into two different types that are staggered and eclipsed conformation. In eclipsed conformation, hydrogens are connected to two carbons closest to each other. In staggered, hydrogens are connected to two carbons as far as possible.
Complete answer:
If a compound has higher energy than that compound will be less stable.
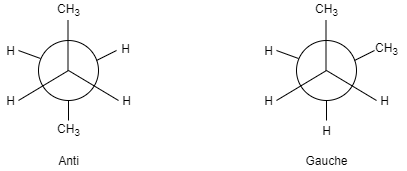
Let us take an example of staggered conformers of butane in which we compare the anti and gauche conformers.
In gauche isomers, there is no interaction between the hydrogen- hydrogen and methyl –hydrogen, hence its energy will be zero but we can see a butane gauche interaction that increases the energy by $0.9KCalmo{{l}^{-1}}$. Therefore, for gauche conformers the energy of the molecule will be $0.9KCalmo{{l}^{-1}}$.
Let us see the structure of gauche conformers-
In anti conformers, there is no interaction between the hydrogen- hydrogen, methyl-hydrogen, methyl-methyl. Hence the molecule energy will be zero.
Let us see the structure of anti conformers-
As we can see here the energy of gauche is much higher than the anti therefore we can say that the stability of anti will be more than gauche.
The stability of the molecule is inversely proportional to the energy of the molecule.
Note: In staggered conformations, the group of atoms are arranged in ${{60}^{{}^\circ }}$ dihedral angle whereas in eclipsed conformations, the group of atoms are arranged in ${{0}^{{}^\circ }}$ dihedral angle.
Staggered conformations show high steadiness whereas eclipsed conformation shows low steadiness.
Complete answer:
If a compound has higher energy than that compound will be less stable.
Let us take an example of staggered conformers of butane in which we compare the anti and gauche conformers.
In gauche isomers, there is no interaction between the hydrogen- hydrogen and methyl –hydrogen, hence its energy will be zero but we can see a butane gauche interaction that increases the energy by $0.9KCalmo{{l}^{-1}}$. Therefore, for gauche conformers the energy of the molecule will be $0.9KCalmo{{l}^{-1}}$.
Let us see the structure of gauche conformers-
In anti conformers, there is no interaction between the hydrogen- hydrogen, methyl-hydrogen, methyl-methyl. Hence the molecule energy will be zero.
Let us see the structure of anti conformers-
As we can see here the energy of gauche is much higher than the anti therefore we can say that the stability of anti will be more than gauche.
The stability of the molecule is inversely proportional to the energy of the molecule.

Note: In staggered conformations, the group of atoms are arranged in ${{60}^{{}^\circ }}$ dihedral angle whereas in eclipsed conformations, the group of atoms are arranged in ${{0}^{{}^\circ }}$ dihedral angle.
Staggered conformations show high steadiness whereas eclipsed conformation shows low steadiness.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




