
D-glucose and D-mannose are:
A. Anomers
B. Enantiomers
C. Geometrical isomers
D. Epimers
Answer
560.4k+ views
Hint: In order to solve these types of questions we should have the knowledge of structural analysis because these are structural based questions which can be simply identified by just looking and then analyzing the structure and here the configuration of H and OH will be different about one carbon.
Complete Answer:
While we are solving this question we should have the structural knowledge:
Since structure is important so first we will make the structure of D-glucose and D-mannose are;
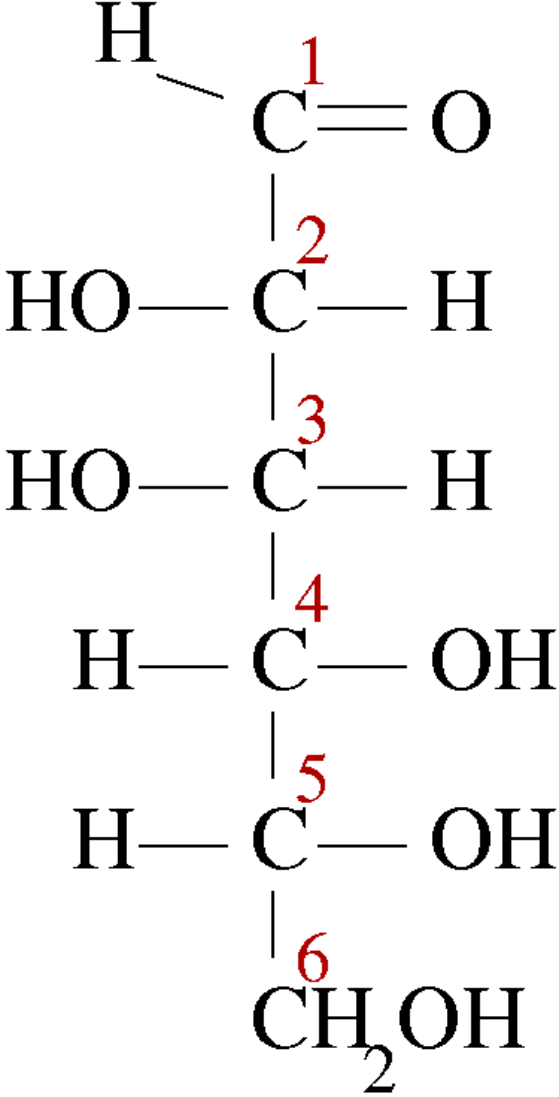
D-Mannose:
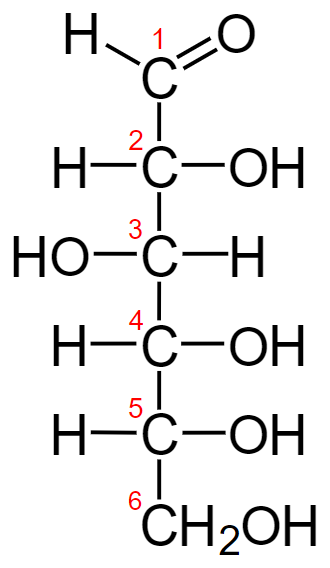
D-Glucose:
So now we will discuss the options one by one so first we are starting with anomers:
Anomers are cyclic monosaccharides or glycosides, which are different from each other in the configuration at C-1 if they are aldoses or in the configuration at C-2 if they are ketoses.
Since we can see that these are even not cyclic so this option is not the appropriate for this so will summarize that these two compounds D-mannose and D-glucose are not anomers.
Now coming to second option we will check for enantiomers:
So enantiomers are chiral molecules that are mirror images of one another. And the molecules should not be superimposable on one one another. So this option is also not satisfactory.
Now coming to third option we will check for geometrical isomers:
So geometrical isomers can be explained as if each of the more than one compounds which are different from each other in the arrangement of groups with respect to the following ;
Double bond, Ring or other rigid structures.
And here in this case not even one can be applied correctly so this option is also not fulfilling the need of question
At the end we will see the epimers ;
While studying stereochemistry we will learn epimers in which two of them have opposite configurations at only one stereogenic center of at least two. All other stereogenic molecules are the same in each. And epimerization is the interconversion of one epimer to the other epimer. So we can see that in this question we can see that around the carbon C-2 there are all the same groups present but in different orientations. So our option will be satisfactory for this question.
Hence the correct answer is option D.
Note: Anomers are also a special types of epimers in which the special condition should be satisfied that is there should be cyclic chain and aldoses should be at C-1 or ketoses should be art C-2 so these conditions are not satisfactory when we will do more such types of questions then we can say it just by a look.
Complete Answer:
While we are solving this question we should have the structural knowledge:
Since structure is important so first we will make the structure of D-glucose and D-mannose are;
D-Mannose:

D-Glucose:

So now we will discuss the options one by one so first we are starting with anomers:
Anomers are cyclic monosaccharides or glycosides, which are different from each other in the configuration at C-1 if they are aldoses or in the configuration at C-2 if they are ketoses.
Since we can see that these are even not cyclic so this option is not the appropriate for this so will summarize that these two compounds D-mannose and D-glucose are not anomers.
Now coming to second option we will check for enantiomers:
So enantiomers are chiral molecules that are mirror images of one another. And the molecules should not be superimposable on one one another. So this option is also not satisfactory.
Now coming to third option we will check for geometrical isomers:
So geometrical isomers can be explained as if each of the more than one compounds which are different from each other in the arrangement of groups with respect to the following ;
Double bond, Ring or other rigid structures.
And here in this case not even one can be applied correctly so this option is also not fulfilling the need of question
At the end we will see the epimers ;
While studying stereochemistry we will learn epimers in which two of them have opposite configurations at only one stereogenic center of at least two. All other stereogenic molecules are the same in each. And epimerization is the interconversion of one epimer to the other epimer. So we can see that in this question we can see that around the carbon C-2 there are all the same groups present but in different orientations. So our option will be satisfactory for this question.
Hence the correct answer is option D.
Note: Anomers are also a special types of epimers in which the special condition should be satisfied that is there should be cyclic chain and aldoses should be at C-1 or ketoses should be art C-2 so these conditions are not satisfactory when we will do more such types of questions then we can say it just by a look.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




