
D-glucose and L-glucose are examples of:
A. diastereomers
B. enantiomers
C. anomers
D. epimers
Answer
576.6k+ views
Hint:D-glucose is formed when glucose rotates the plane polarized light in the right direction (dextrorotation) and L-glucose is formed when glucose rotates the plane polarized light in the left direction (levorotation). The D-glucose and L-glucose is non-superimposable mirror image of each other.
Complete step by step answer:
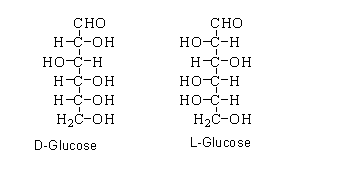
When the hydroxyl group present on the carbon atom of forth position and fifth position is localized at the right side of the fischer projection. Then the glucose shows D-configuration.
When the hydroxyl group present on the carbon atom of forth position and fifth position is localized at the left side of the fischer projection. Then the glucose shows L-configuration.
Enantiomers are classified as chiral molecules which are mirror images of each other. The molecules cannot overlap each other to form a single molecule, this means that enantiomers are non-superimposable on each other. The chiral molecules which contain one or more than one stereocenters can form enantiomers.
As can be seen that the D-form of glucose differs from the L-form on the basis of the hydroxyl group attached to the fourth position of carbon and fifth position of carbon. When they are placed upside down, they do not form the single molecule.
D-glucose and L-glucose are examples of enantiomers
Therefore, the correct option is B.
Note:
When the enantiomers are flipped to 180 degree using the Wedge-Dash notation, the group or atom which are attached to the wedged bond become dashed and the group or atom attached to the dashed bond become wedged.
Complete step by step answer:
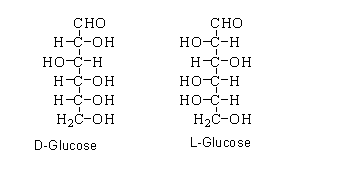
When the hydroxyl group present on the carbon atom of forth position and fifth position is localized at the right side of the fischer projection. Then the glucose shows D-configuration.
When the hydroxyl group present on the carbon atom of forth position and fifth position is localized at the left side of the fischer projection. Then the glucose shows L-configuration.
Enantiomers are classified as chiral molecules which are mirror images of each other. The molecules cannot overlap each other to form a single molecule, this means that enantiomers are non-superimposable on each other. The chiral molecules which contain one or more than one stereocenters can form enantiomers.
As can be seen that the D-form of glucose differs from the L-form on the basis of the hydroxyl group attached to the fourth position of carbon and fifth position of carbon. When they are placed upside down, they do not form the single molecule.
D-glucose and L-glucose are examples of enantiomers
Therefore, the correct option is B.
Note:
When the enantiomers are flipped to 180 degree using the Wedge-Dash notation, the group or atom which are attached to the wedged bond become dashed and the group or atom attached to the dashed bond become wedged.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




