
Diamond is the hardest allotrope of carbon. Give reason for its hardness.
Answer
594k+ views
Hint: Carbon has well known allotropes like graphite and diamond. It is very interesting to know that both are made up of the same element, carbon, yet diamond is the hardest known mineral whereas graphite is very soft. The reason lies in the physical structure of the two.
Complete answer:
Allotropes of an element are the structurally different forms of the same element. They have the same atoms of the element but the structures in which these atoms are arranged vary greatly. This results in various physical forms of the same element. Allotropes differ in physical as well as chemical properties even though they are composed of molecules of the same element. The structure of graphite and diamond are shown below;
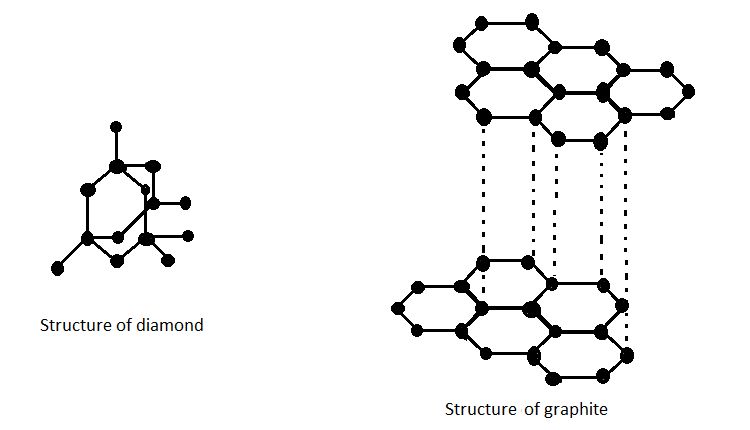
Each carbon atom in a diamond is covalently bonded to four other carbons forming a tetrahedral structure. These tetrahedrons together form a 3-dimensional network of carbon rings. This stable network of covalent bonds and hexagonal rings makes diamond the hardest allotrope of carbon and also the hardest mineral. Graphite has covalent structure where each carbon atom is joined to three carbon atoms by covalent bond. The carbon atoms form layers with a hexagonal arrangement of atoms.
Carbon is known to form many allotropes due to its valency. There are 8 allotropes of carbon, the most common being diamond and graphite.
Note: Contrast to diamond, graphite is a very soft allotrope of carbon. It is dull and dark unlike diamond which is known for its shining and luster. However, graphite can conduct electricity and diamond cannot. The reason lies again in the difference of structure between the two.
Complete answer:
Allotropes of an element are the structurally different forms of the same element. They have the same atoms of the element but the structures in which these atoms are arranged vary greatly. This results in various physical forms of the same element. Allotropes differ in physical as well as chemical properties even though they are composed of molecules of the same element. The structure of graphite and diamond are shown below;

Each carbon atom in a diamond is covalently bonded to four other carbons forming a tetrahedral structure. These tetrahedrons together form a 3-dimensional network of carbon rings. This stable network of covalent bonds and hexagonal rings makes diamond the hardest allotrope of carbon and also the hardest mineral. Graphite has covalent structure where each carbon atom is joined to three carbon atoms by covalent bond. The carbon atoms form layers with a hexagonal arrangement of atoms.
Carbon is known to form many allotropes due to its valency. There are 8 allotropes of carbon, the most common being diamond and graphite.
Note: Contrast to diamond, graphite is a very soft allotrope of carbon. It is dull and dark unlike diamond which is known for its shining and luster. However, graphite can conduct electricity and diamond cannot. The reason lies again in the difference of structure between the two.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




