
What is the difference between acetal and ketal?
Answer
524.1k+ views
Hint : As from the names we can conclude that acetal form is related to aldehydes and the ketal form is related to ketones. There are other forms of acetal and ketals present in nature. These are known as hemiacetals and hemiketals. These compounds play the great role in organic chemistry mainly in aldehydes and ketones reactions.
Complete Step By Step Answer:
The structure of acetal and ketal are shown here:
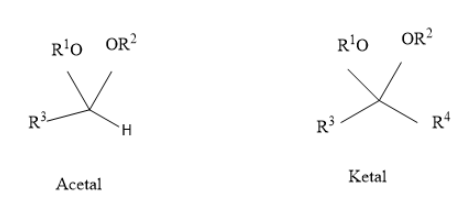
From the above diagram we can see that there is one H atom and two ketonic group and one alkyl chain is present in the compound called acetal and in the ketal two ketonic groups and two alkyl chains are present which is the major difference between them. In acetal there should be one hydrogen atom that has to be attached directly with the central carbon. Acetal and ketal are formed when hemiacetals and hemiketals react with a second alcohol nucleophile. Some examples of acetals are benzylidene acetal, dimethoxymethane, dioxolane, metaldehyde and paraldehyde, etc. whereas examples of ketals are mostly ketonic compounds having structure as mentioned above.
Note :
In acetal ${R^1}$ and ${R^2}$ can be same or different. The central carbon atom has four bonds to it. Therefore, it is saturated and is tetrahedral in shape. An acetal is formed when the hydroxyl group of a hemiacetal becomes protonated and is lost as water. The carbocation which is produced is attacked by a molecule of alcohol and loss of proton from the attached alcohol gives the acetal.
Complete Step By Step Answer:
The structure of acetal and ketal are shown here:
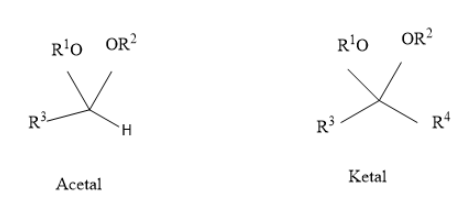
From the above diagram we can see that there is one H atom and two ketonic group and one alkyl chain is present in the compound called acetal and in the ketal two ketonic groups and two alkyl chains are present which is the major difference between them. In acetal there should be one hydrogen atom that has to be attached directly with the central carbon. Acetal and ketal are formed when hemiacetals and hemiketals react with a second alcohol nucleophile. Some examples of acetals are benzylidene acetal, dimethoxymethane, dioxolane, metaldehyde and paraldehyde, etc. whereas examples of ketals are mostly ketonic compounds having structure as mentioned above.
Note :
In acetal ${R^1}$ and ${R^2}$ can be same or different. The central carbon atom has four bonds to it. Therefore, it is saturated and is tetrahedral in shape. An acetal is formed when the hydroxyl group of a hemiacetal becomes protonated and is lost as water. The carbocation which is produced is attacked by a molecule of alcohol and loss of proton from the attached alcohol gives the acetal.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




