
What is the difference between isopentane and neopentane structures?
A.) Iso has one $\text{C}{{\text{H}}_{\text{3}}}$ group and neo has two $\text{C}{{\text{H}}_{\text{3}}}$ on the C atom next to end C atom.
B.) lso has two $\text{C}{{\text{H}}_{\text{3}}}$ group and neo has two $\text{C}{{\text{H}}_{\text{3}}}$ on the C atom next to end C atom.
C.) Iso has two $\text{C}{{\text{H}}_{\text{3}}}$ group and neo has three $\text{C}{{\text{H}}_{\text{3}}}$ on the C atom next to end C atom
D.) None of the above.
Answer
606.9k+ views
Hint: We know that isopentane contains a four-membered carbon chain while neopentane contains one carbon centre attached with four methyl groups. Knowing this fact we can solve the following question.
Complete step-by-step answer:
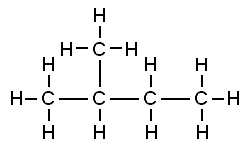
Isopentane is a compound having 5 carbon atoms with only a single branched chain. Neopentane is a compound which has 5 carbon atoms with two or more than two branched chains. The difference between isopentane and neopentane structure is that isopentane consists of a four-membered carbon chain with a single methyl group that was attached to this chain at the second carbon atom. On the other hand, neopentane contains four methyl groups attached to a carbon atom at its centre.
Isopentane
Neopentane Therefore, the answer does not match any of the given options.
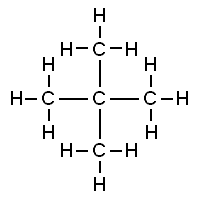
Hence, the correct option is Option D.
Note: We know that isopentane and neopentane both are an organic compound having the chemical formula ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{12}}}$. Isopentane exists as a colourless liquid while Neopentane exists as a colourless gas, at standard temperature and pressure. The IUPAC name of Isopentane is 2-Methylbutane whereas the IUPAC name of Neopentane is 2,2-Dimethylpropane. The molar mass of Isopentane is \[\text{72}\text{.15 g/mol}\] and its melting point is \[\text{-161 }\!\!{}^\circ\!\!\text{ C to 159 }\!\!{}^\circ\!\!\text{ C}\] and its boiling point ranges from \[\text{27}\text{.8}{}^\circ \text{C to 28}\text{.2}{}^\circ \text{C}\]. Neopentane is a highly volatile liquid and its melting point is \[\text{-16}\text{.5 }\!\!{}^\circ\!\!\text{ C}\] and its boiling point is \[\text{9}\text{.5 }\!\!{}^\circ\!\!\text{ C}\].
Complete step-by-step answer:

Isopentane is a compound having 5 carbon atoms with only a single branched chain. Neopentane is a compound which has 5 carbon atoms with two or more than two branched chains. The difference between isopentane and neopentane structure is that isopentane consists of a four-membered carbon chain with a single methyl group that was attached to this chain at the second carbon atom. On the other hand, neopentane contains four methyl groups attached to a carbon atom at its centre.
Isopentane
Neopentane Therefore, the answer does not match any of the given options.

Hence, the correct option is Option D.
Note: We know that isopentane and neopentane both are an organic compound having the chemical formula ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{12}}}$. Isopentane exists as a colourless liquid while Neopentane exists as a colourless gas, at standard temperature and pressure. The IUPAC name of Isopentane is 2-Methylbutane whereas the IUPAC name of Neopentane is 2,2-Dimethylpropane. The molar mass of Isopentane is \[\text{72}\text{.15 g/mol}\] and its melting point is \[\text{-161 }\!\!{}^\circ\!\!\text{ C to 159 }\!\!{}^\circ\!\!\text{ C}\] and its boiling point ranges from \[\text{27}\text{.8}{}^\circ \text{C to 28}\text{.2}{}^\circ \text{C}\]. Neopentane is a highly volatile liquid and its melting point is \[\text{-16}\text{.5 }\!\!{}^\circ\!\!\text{ C}\] and its boiling point is \[\text{9}\text{.5 }\!\!{}^\circ\!\!\text{ C}\].
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




