
Differentiate between enantiomer and diastereomer.
Answer
587.7k+ views
Hint: Think about the definitions of the given terms with respect to the chiral centers that are involved and the difference in the placement of the functional groups around those chiral centers.
Complete step by step answer:
Let us understand the definition of chirality or a chiral carbon before moving on to the differences between the types of isomers given. A chiral carbon is an asymmetrical carbon that is attached to four different functional groups. The number of chiral centers or carbons will determine how many isomers the molecule will have.
First, we will look at the differences between the given terms: enantiomers and diastereomers and then we will look at some examples that will provide clarity.
Now, we will look at some examples of enantiomers and diastereomers for further clarity.
- Enantiomers
All the different types of D- and L- forms of molecules are enantiomers of each other. We will look at the compounds glucose and mannose.
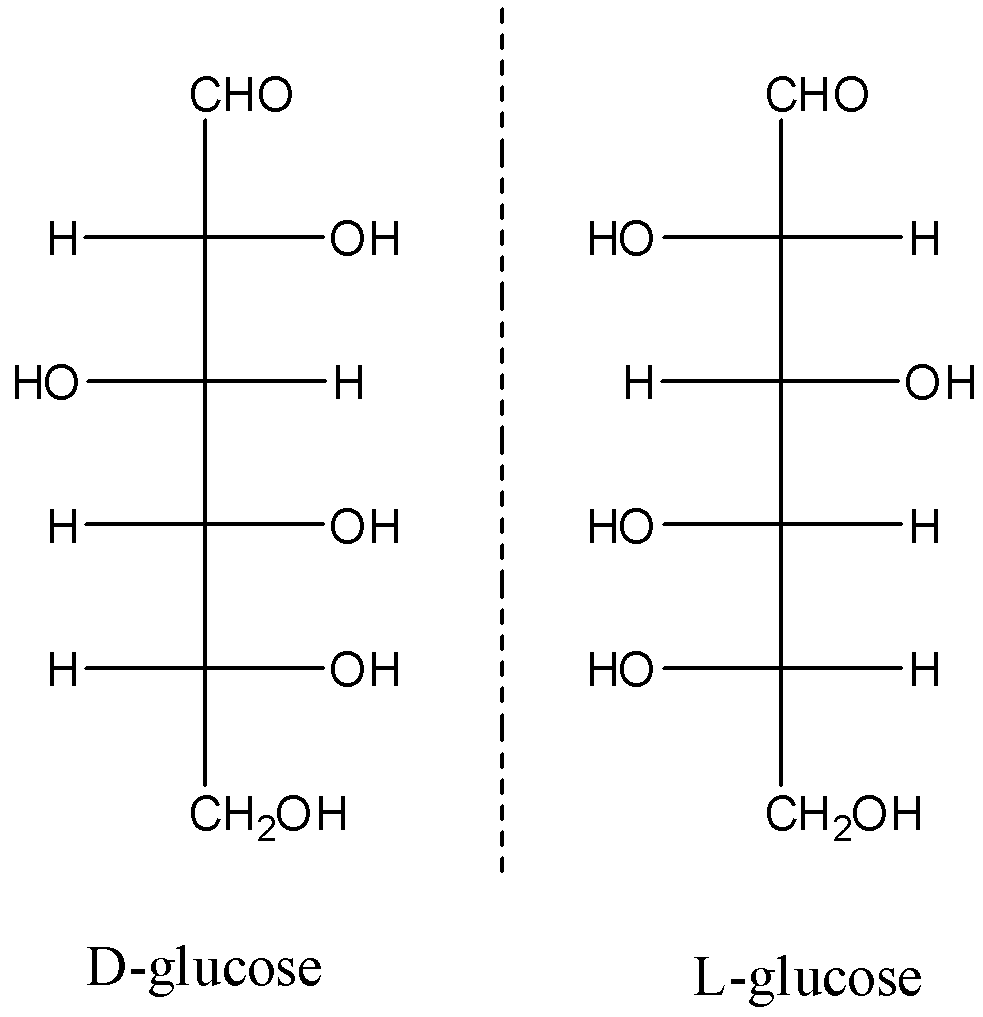
Here, we can see that the given forms of glucose are non-superimposable mirror images of each other.
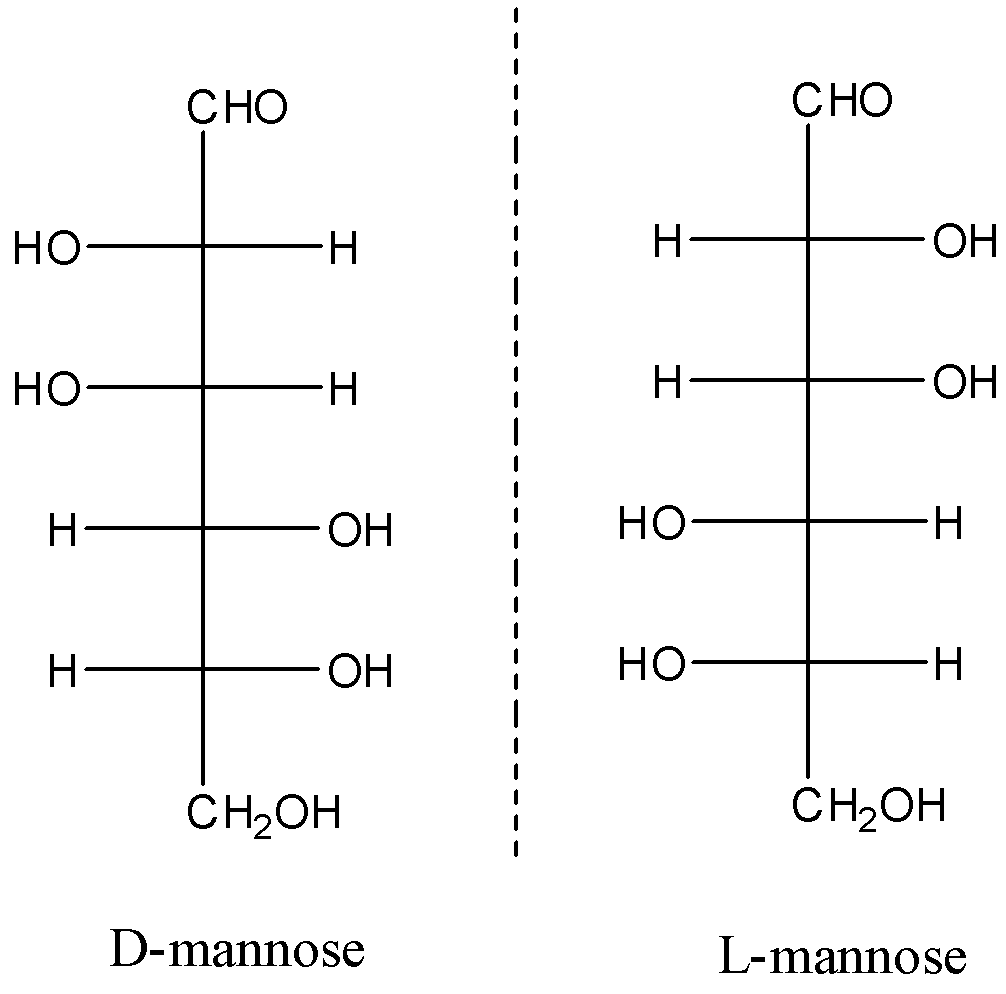
Here, we can see that the given forms of mannose are non-superimposable mirror images of each other.
Now, let us consider the D- forms of glucose and altrose.
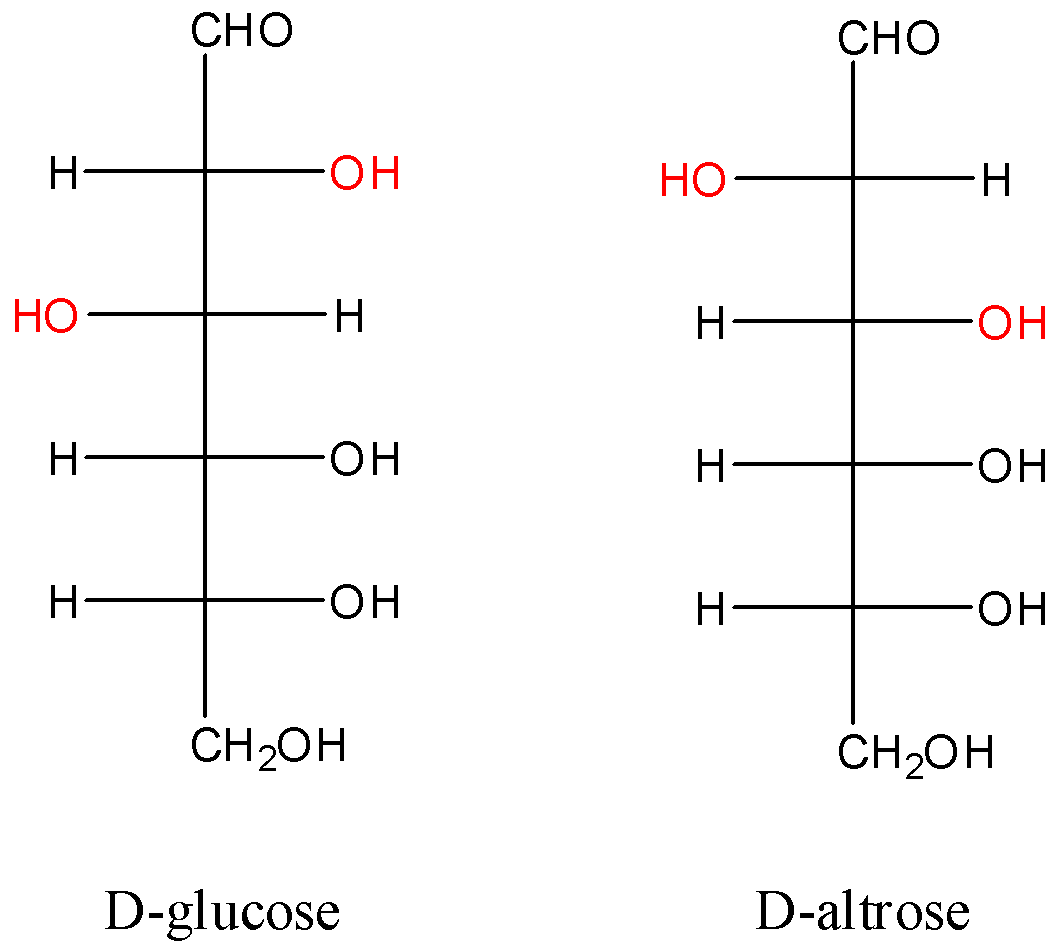
Here, we can see that the position of the $-OH$ group differs at two stereocenters on the aldohexose. So, we can say that they are diastereomers of each other.
Note: Compare the structures of D-glucose and D-mannose, you will notice that only one of the stereocenters or the chiral carbon differ in the positioning of the $-OH$ group. Such isomers are called epimers. In epimers, the stereochemistry is different at only one of the chiral centers. We can also say that D-glucose and L-mannose are diastereomers of each other since they differ at three chiral centers and not all.
Complete step by step answer:
Let us understand the definition of chirality or a chiral carbon before moving on to the differences between the types of isomers given. A chiral carbon is an asymmetrical carbon that is attached to four different functional groups. The number of chiral centers or carbons will determine how many isomers the molecule will have.
First, we will look at the differences between the given terms: enantiomers and diastereomers and then we will look at some examples that will provide clarity.
| Enantiomer | Diastereomer |
| Enantiomers are non-superimposable mirror images of each other. | Diastereomers are not mirror images of each other and are not superimposable. |
| The stereochemistry at each chiral center is different. | The stereochemistry at more than one, but not all centers is different. |
| They are always present in pairs. | The number of diastereomers of a molecule depends on the number of chiral carbons it has. |
| Enantiomers are always optically active. | They may or may not be optically active. |
| Classified as D- or L- enantiomers which indicate how the plane polarized light will rotate | Do not have a special system for classification and can be named using the R- and S- rules for each chiral atom. |
| All properties are the same except for the ability to rotate plane polarized light in different directions. | The physical and chemical properties differ for each diastereomer. |
| Cannot be separated using simple physical methods. | Can be separated using methods like recrystallization and distillation due to differences in boiling points. |
Now, we will look at some examples of enantiomers and diastereomers for further clarity.
- Enantiomers
All the different types of D- and L- forms of molecules are enantiomers of each other. We will look at the compounds glucose and mannose.

Here, we can see that the given forms of glucose are non-superimposable mirror images of each other.

Here, we can see that the given forms of mannose are non-superimposable mirror images of each other.
Now, let us consider the D- forms of glucose and altrose.

Here, we can see that the position of the $-OH$ group differs at two stereocenters on the aldohexose. So, we can say that they are diastereomers of each other.
Note: Compare the structures of D-glucose and D-mannose, you will notice that only one of the stereocenters or the chiral carbon differ in the positioning of the $-OH$ group. Such isomers are called epimers. In epimers, the stereochemistry is different at only one of the chiral centers. We can also say that D-glucose and L-mannose are diastereomers of each other since they differ at three chiral centers and not all.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




