
Dipole moment of (1) p-dinitrobenzene (2) p-dichlorobenzene (3) p-dimethoxybenzene are in the order:
(a) 3 > 2 > 1
(b) 3 = 2 > 1
(c) 3=2=1
(d) 3 > (2 =1)
Answer
560.1k+ views
Hint: The dipole moment is formed when there is electronegativity difference between the atoms. The dipole moment measures the compound's polarity. When the moment of dipole is towards the pair of electrons, then the dipole moment is larger.
Complete step by step answer:
The structure of p-dinitrobenzene is shown below.
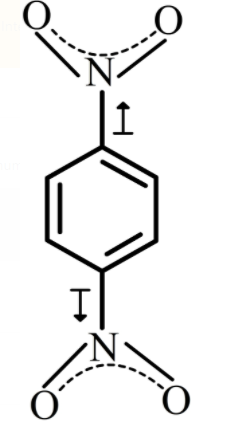
There is a dipole moment between the carbon atom of benzene ring and nitrogen atom containing lone pairs of electrons. As nitrogen is more electronegative than hydrogen, then the moment of dipole is towards the nitrogen atom. As there are two nitro groups attached to the benzene ring, the dipole moment generated by both the nitro groups will cancel out each other and the net dipole moment will be 0.
The structure of p-dichlorobenzene.is shown below.
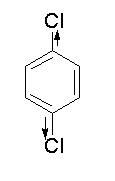
There is a dipole moment between the carbon atom of benzene ring and chlorine atom containing lone pairs of electrons. As chlorine is more electronegative than hydrogen, then the moment of dipole is towards the chlorine atom. As there are two chlorine atoms attached to the benzene ring, the dipole moment generated by both the two chlorine atoms will cancel out each other and the net dipole moment will be 0.
The structure of p-dimethoxybenzene is shown below.
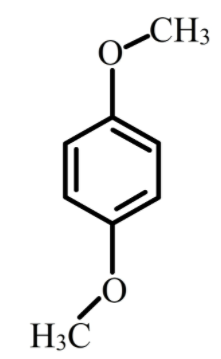
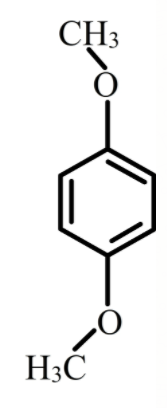
The p-dimethoxybenzene exists mainly in two forms, one is cis p-dimethoxybenzene and trans p-methoxybenzene. As trans is a symmetrical molecule its dipole moment is 0 while cis form is not symmetrical molecule therefore, its dipole moment is 2.25 D.
Thus, dipole moments of (1) p-dinitrobenzene (2) p-dichlorobenzene (3) p-dimethoxybenzene are in the order 3 > (2 =1).
Therefore, the correct option is D.
Note:
Make sure if the molecule exists in different forms or not because the symmetry of the molecule changes the dipole moment in the molecule.
Complete step by step answer:
The structure of p-dinitrobenzene is shown below.

There is a dipole moment between the carbon atom of benzene ring and nitrogen atom containing lone pairs of electrons. As nitrogen is more electronegative than hydrogen, then the moment of dipole is towards the nitrogen atom. As there are two nitro groups attached to the benzene ring, the dipole moment generated by both the nitro groups will cancel out each other and the net dipole moment will be 0.
The structure of p-dichlorobenzene.is shown below.

There is a dipole moment between the carbon atom of benzene ring and chlorine atom containing lone pairs of electrons. As chlorine is more electronegative than hydrogen, then the moment of dipole is towards the chlorine atom. As there are two chlorine atoms attached to the benzene ring, the dipole moment generated by both the two chlorine atoms will cancel out each other and the net dipole moment will be 0.
The structure of p-dimethoxybenzene is shown below.


The p-dimethoxybenzene exists mainly in two forms, one is cis p-dimethoxybenzene and trans p-methoxybenzene. As trans is a symmetrical molecule its dipole moment is 0 while cis form is not symmetrical molecule therefore, its dipole moment is 2.25 D.
Thus, dipole moments of (1) p-dinitrobenzene (2) p-dichlorobenzene (3) p-dimethoxybenzene are in the order 3 > (2 =1).
Therefore, the correct option is D.
Note:
Make sure if the molecule exists in different forms or not because the symmetry of the molecule changes the dipole moment in the molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




