
Dipole moment of $N{{H}_{3}}$ is ________ (more/less) than $N{{F}_{3}}$
Answer
542.1k+ views
Hint: Think about what dipole moment actually is. Dipole moment is seen almost everywhere where there is a difference in charges/polarity and there is a significant distance between them. The most common dipole you have seen is a bar magnet.
Complete answer:
Let us start by knowing what dipole moment actually is. Dipole moment is defined as the product of the charge and the perpendicular or shortest distance between the charges. It is a scalar quantity that means, dipole moment has no direction associated with it.
Mathematically,
\[\mu \text{ }=\text{ }Q\text{ }\times \text{ }d\], where Q is the charge and d is the perpendicular distance
Dipole moment is used in many places. For example, dipole moment is used to find out whether a compound is polar or not. Any compound having non-zero dipole moment is said to be polar. More the dipole moment, more the polarity of the bond and more polarity means stronger bond.
Dipole moment can be used to find out the percentage of ionic character in a compound. A compound is said to be more ionic if it has more dipole moment. The formula for finding ionic character is:
\[[\mu \left( real \right)\div \mu \left( observed \right)]\times 100%\]
It is observed that molecules which have a plane of symmetry have 0 net dipole moment. It is so because as the dipole moment is scalar quantity, they get cancelled out in different directions.
The geometrical shape of both the molecules are pyramidal in shape. In both the molecules N has one lone pair .
Now F is more electronegative than H and N- F bond is more polar than N- H bond.
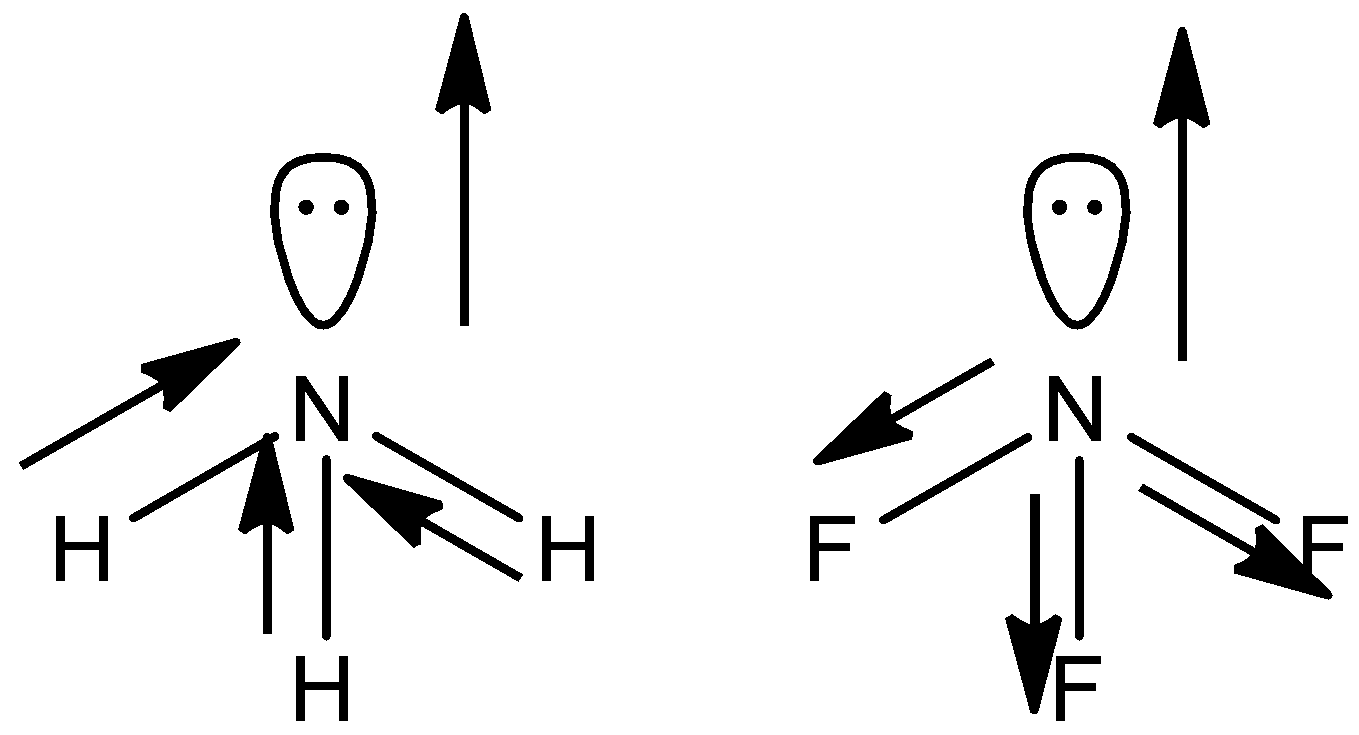
Now, in the case of ammonia, the direction of the lone pair dipole moment and the bond pair dipole moment is the same whereas in case of $N{{F}_{3}}$ it is opposite in direction.
So in ammonia molecules individual dipole moment vectors add each other whereas in $N{{F}_{3}}$ they cancel each other.
So, ammonia has more dipole.
More the dipole moment, more the bond strength and more the boiling point of the substance. Generally compounds which are ionic in nature have a high boiling point and are less volatile.
Note:
Do not get confused with the direction of the dipole moment. Dipole moment is a scalar quantity and does not have any direction. But once it is a scalar quantity, it can get added or subtracted. For example, in the molecule \[B{{H}_{3}}\], dipoles cancel out each their and resultant dipole is less, but in case of \[_{{}}N{{H}_{3}}\], dipoles add to each other, as they are in the same direction and resultant dipole is not 0.
Complete answer:
Let us start by knowing what dipole moment actually is. Dipole moment is defined as the product of the charge and the perpendicular or shortest distance between the charges. It is a scalar quantity that means, dipole moment has no direction associated with it.
Mathematically,
\[\mu \text{ }=\text{ }Q\text{ }\times \text{ }d\], where Q is the charge and d is the perpendicular distance
Dipole moment is used in many places. For example, dipole moment is used to find out whether a compound is polar or not. Any compound having non-zero dipole moment is said to be polar. More the dipole moment, more the polarity of the bond and more polarity means stronger bond.
Dipole moment can be used to find out the percentage of ionic character in a compound. A compound is said to be more ionic if it has more dipole moment. The formula for finding ionic character is:
\[[\mu \left( real \right)\div \mu \left( observed \right)]\times 100%\]
It is observed that molecules which have a plane of symmetry have 0 net dipole moment. It is so because as the dipole moment is scalar quantity, they get cancelled out in different directions.
The geometrical shape of both the molecules are pyramidal in shape. In both the molecules N has one lone pair .
Now F is more electronegative than H and N- F bond is more polar than N- H bond.

Now, in the case of ammonia, the direction of the lone pair dipole moment and the bond pair dipole moment is the same whereas in case of $N{{F}_{3}}$ it is opposite in direction.
So in ammonia molecules individual dipole moment vectors add each other whereas in $N{{F}_{3}}$ they cancel each other.
So, ammonia has more dipole.
More the dipole moment, more the bond strength and more the boiling point of the substance. Generally compounds which are ionic in nature have a high boiling point and are less volatile.
Note:
Do not get confused with the direction of the dipole moment. Dipole moment is a scalar quantity and does not have any direction. But once it is a scalar quantity, it can get added or subtracted. For example, in the molecule \[B{{H}_{3}}\], dipoles cancel out each their and resultant dipole is less, but in case of \[_{{}}N{{H}_{3}}\], dipoles add to each other, as they are in the same direction and resultant dipole is not 0.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




