
Discuss the nature of bonding in metal carbonyls.
Answer
583.8k+ views
Hint:
We know that the metal carbonyls are the compound in which coordination complexes are formed and the transition metals combine with the carbon monoxide (CO). They are organometallic compounds.
Complete step by step solution
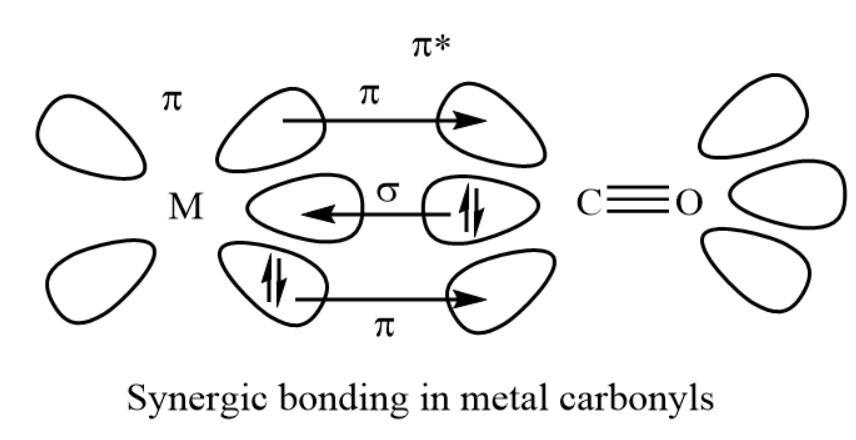
As we all know, the metal carbonyl bond is the bond between the metal and the carbon. This bond shows both the sigma and pi characters. A sigma bond generally is made when the carbonyl carbon present in the complex gives an electrons’ lone pairs to the unoccupied orbital of the given metal. A pi bond usually made by the donation or contribution of an electrons’ pair from the occupied metal.
Metal carbonyls show the two types of bonding.
- The formation of sigma bond from carbonyl to metal that is (\[{\rm{M}} \leftarrow {\rm{C}}\]) by the donation of electrons’ lone pair of carbon which is present in carbonyl group into unfilled orbital of the given metal atom. This is one of the dative overlaps.
- The formation of pi bond from metal to carbonyl that is (\[{\rm{M}} \to {\rm{C}}\]) by the donation of electrons from complete metal ${\rm{d}}$-orbitals into unfilled antibonding ${\rm{\pi *}}$ molecular orbitals of the carbonyl group.
- The back bonding produces a synergic effect and it makes stronger the bond between carbonyl and the metal.
Note:
The use of metal carbonyls is written below.
- The metal carbonyls are used as the catalyst in the synthesis of other chemical species.
- The metal carbonyls are used in the chemistry laboratory.
We know that the metal carbonyls are the compound in which coordination complexes are formed and the transition metals combine with the carbon monoxide (CO). They are organometallic compounds.
Complete step by step solution
As we all know, the metal carbonyl bond is the bond between the metal and the carbon. This bond shows both the sigma and pi characters. A sigma bond generally is made when the carbonyl carbon present in the complex gives an electrons’ lone pairs to the unoccupied orbital of the given metal. A pi bond usually made by the donation or contribution of an electrons’ pair from the occupied metal.
Metal carbonyls show the two types of bonding.
- The formation of sigma bond from carbonyl to metal that is (\[{\rm{M}} \leftarrow {\rm{C}}\]) by the donation of electrons’ lone pair of carbon which is present in carbonyl group into unfilled orbital of the given metal atom. This is one of the dative overlaps.
- The formation of pi bond from metal to carbonyl that is (\[{\rm{M}} \to {\rm{C}}\]) by the donation of electrons from complete metal ${\rm{d}}$-orbitals into unfilled antibonding ${\rm{\pi *}}$ molecular orbitals of the carbonyl group.
- The back bonding produces a synergic effect and it makes stronger the bond between carbonyl and the metal.

Note:
The use of metal carbonyls is written below.
- The metal carbonyls are used as the catalyst in the synthesis of other chemical species.
- The metal carbonyls are used in the chemistry laboratory.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




