
Distinguish between electrophile and nucleophile.
Answer
582.9k+ views
Hint: Both of these species are related to the donation or gaining of electrons. One is an electron acceptor and on the other hand the other is an electron donor.
Complete step by step answer:
Electrophiles are basically known as electron loving species. If we break the word electro is from electrons and phile means loving. Basically, an electron is a reagent that is characterized with a low density of electrons in its valence shell and therefore reacts with a high density molecule, ion or any atom to form the covalent bond. An electrophile easily gets detected by a positive charge with empty orbitals. As you know, electrons move from the area of high density to the area of low density and the unlike charges seem to attract each other. This concept explains the attraction of electrons by the electrophile atoms, molecules or ions. An electrophile as defined above can also be called as Lewis acid because, same as Lewis acid, it accepts electrons.
EXAMPLE: ${\text{B}}{{\text{r}}^{\text{ + }}}{\text{,C}}{{\text{l}}^{\text{ + }}}$
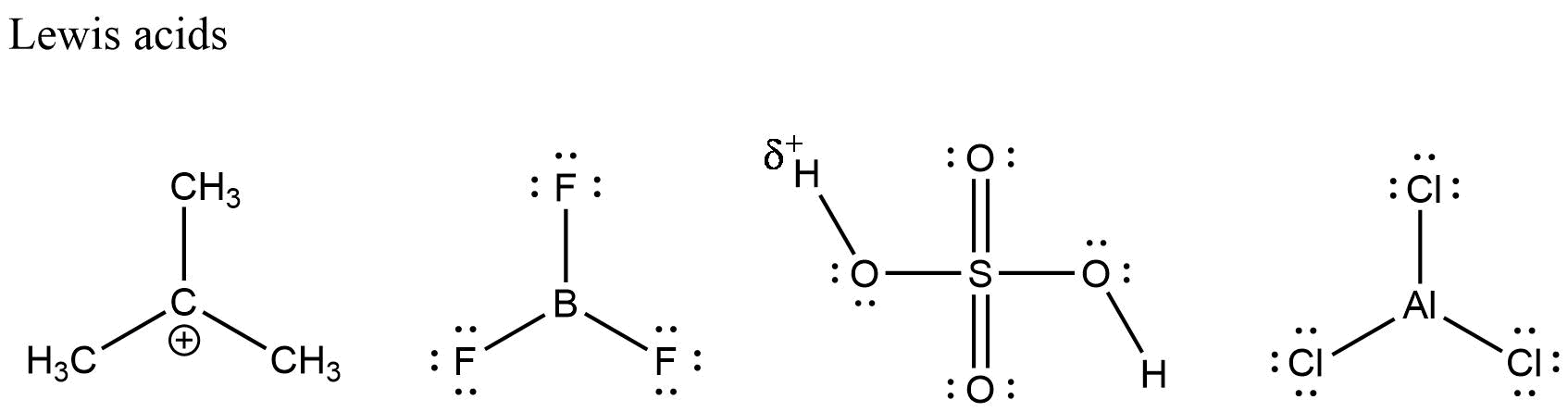
Nucleophiles are opposite to electrophiles. These are nucleus loving species. Nucleo in word nucleophile refers to nucleus and phile means loving. According to the definition, these are rich in electrons but as they are nucleus loving they donate electrons easily to electrophiles that results in the formation of covalent bonds. These species can be identified with lone pairs, pi bonds and negative charges. If electrophiles are Lewis acid then nucleophiles are Lewis base because they donate electrons and accept protons. donate electrons and accept protons.
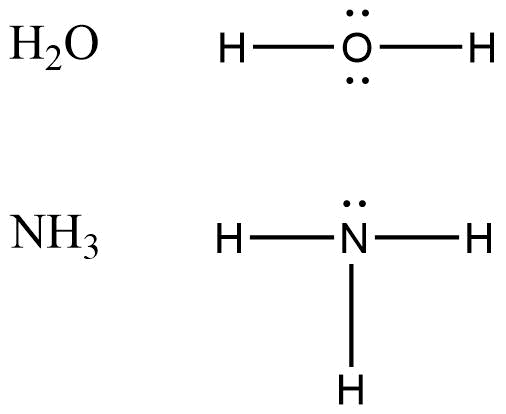
The above two pictures show the examples of electrophiles and nucleophiles.
Note:
Nucleophiles are nucleus loving but actually they are rich in electrons whereas electrophiles are electron loving but they are deficient of electrons. This is the basic difference between electrophiles and nucleophiles.
Complete step by step answer:
Electrophiles are basically known as electron loving species. If we break the word electro is from electrons and phile means loving. Basically, an electron is a reagent that is characterized with a low density of electrons in its valence shell and therefore reacts with a high density molecule, ion or any atom to form the covalent bond. An electrophile easily gets detected by a positive charge with empty orbitals. As you know, electrons move from the area of high density to the area of low density and the unlike charges seem to attract each other. This concept explains the attraction of electrons by the electrophile atoms, molecules or ions. An electrophile as defined above can also be called as Lewis acid because, same as Lewis acid, it accepts electrons.
EXAMPLE: ${\text{B}}{{\text{r}}^{\text{ + }}}{\text{,C}}{{\text{l}}^{\text{ + }}}$

Nucleophiles are opposite to electrophiles. These are nucleus loving species. Nucleo in word nucleophile refers to nucleus and phile means loving. According to the definition, these are rich in electrons but as they are nucleus loving they donate electrons easily to electrophiles that results in the formation of covalent bonds. These species can be identified with lone pairs, pi bonds and negative charges. If electrophiles are Lewis acid then nucleophiles are Lewis base because they donate electrons and accept protons. donate electrons and accept protons.

The above two pictures show the examples of electrophiles and nucleophiles.
Note:
Nucleophiles are nucleus loving but actually they are rich in electrons whereas electrophiles are electron loving but they are deficient of electrons. This is the basic difference between electrophiles and nucleophiles.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE

Calculate the equivalent resistance between a and b class 12 physics CBSE

How many states of matter are there in total class 12 chemistry CBSE

Which of the following is the best conductor of electricity class 12 physics CBSE




