
Distinguish between hexagonal close packing and cubic close packing.
Answer
531.4k+ views
Hint- In order to deal with this question first we have to understand hexagonal close packing and cubic close packing, then we will find the difference between both the packing. We can also find the difference between the both the types of packing on the basis of their structure.
Complete answer:
Hexagonal close packing: In a hexagonal close-packed (hcp) arrangement of atoms, the unit cell consists of three layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon.
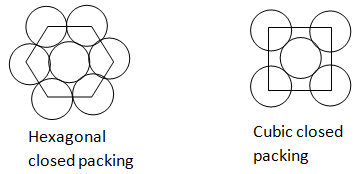
When the tetrahedral voids of the second layer are covered by the spheres of the third layer. So, that the spheres of the third layer are exactly aligned with those of the first layer, we get a pattern of spheres which is repeated in alternate layers. This pattern can be written in the form of ABAB..... pattern. This structure is called hexagonal closed packing (HCP).
Cubic close packing: Cubic Close packing, are known to be the densest possible packings of equal spheres. Simple cubic packing consists of placing spheres centered on integer coordinates in Cartesian space.
When the third layer is placed above the second layer in a manner such that its sphere covers the octahedral voids and the sphere of the third layer is not aligned with the first or the second layer, the arrangement is called ′ C ′ type. This pattern is written as ABCABC........ This structure is called cubic closed packing (CCP).
Note- Matters exist in solid state because of close packing of their constituent particles. Packing of spheres can describe the solid structures of crystals. In a crystal structure, the centers of atoms, ions, or molecules lie on the lattice points. Atoms are assumed to be spherical to explain the bonding and structures of metallic crystals. The arrangements of the spheres are densely packed in order to take up the greatest amount of space possible.
Complete answer:
Hexagonal close packing: In a hexagonal close-packed (hcp) arrangement of atoms, the unit cell consists of three layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon.

When the tetrahedral voids of the second layer are covered by the spheres of the third layer. So, that the spheres of the third layer are exactly aligned with those of the first layer, we get a pattern of spheres which is repeated in alternate layers. This pattern can be written in the form of ABAB..... pattern. This structure is called hexagonal closed packing (HCP).
Cubic close packing: Cubic Close packing, are known to be the densest possible packings of equal spheres. Simple cubic packing consists of placing spheres centered on integer coordinates in Cartesian space.
When the third layer is placed above the second layer in a manner such that its sphere covers the octahedral voids and the sphere of the third layer is not aligned with the first or the second layer, the arrangement is called ′ C ′ type. This pattern is written as ABCABC........ This structure is called cubic closed packing (CCP).
Note- Matters exist in solid state because of close packing of their constituent particles. Packing of spheres can describe the solid structures of crystals. In a crystal structure, the centers of atoms, ions, or molecules lie on the lattice points. Atoms are assumed to be spherical to explain the bonding and structures of metallic crystals. The arrangements of the spheres are densely packed in order to take up the greatest amount of space possible.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




