
How would you distinguish between ortho and para nitrophenol using infrared spectroscopy?
Answer
558.9k+ views
Hint: As we know that an infrared spectroscopy results in peaks of the structures observed which tells us about different bonding between the atoms and ortho and para nitrophenol possess the carbon-carbon double bonds, carbon-hydrogen and carbon-oxygen bonds etc.
Complete step-by-step answer:
As we know that the structures of ortho- nitrophenol can be given as shown below:
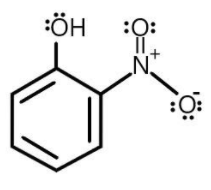
And the structure of para-nitrophenol is given as shown below:

Now, we also know that infrared spectroscopy results are observed on the basis of the peak seen in graphs provided for the particular molecule. Now the ortho and nitrophenol structures as we can see possess carbon-carbon single bonds and double bonds, carbon and hydrogen single bonds, carbon and oxygen single bonds, oxygen and hydrogen single bond and nitro group.
So, in case of ortho phenol, the carbon-carbon double bonds are observed to peak at \[1610 \sim 1590c{m^{ - 1}}\] which are generally medium to weak and sharp in appearance. Similarly, the carbon-hydrogen bonds are shown at peak at $3445,3240c{m^{ - 1}}$ respectively which are medium to weak and sharp in appearance.
And the carbon and oxygen bonds are shown at a peak of about $1300 \sim 1000c{m^{ - 1}}$ and these are strong and sharp in appearance whereas the alcoholic bonds are shown at $3650 \sim 3200c{m^{ - 1}}$ and are medium to strong and broad in appearance. Lastly, the nitro group which is shown at $1570,1310c{m^{ - 1}}$ and is strong and sharp in appearance.
And in case of para nitrophenol, the alcoholic bonds are shown as superimposed by carbon-hydrogen bonds at a peak of about $3365c{m^{ - 1}}$, the carbon-carbon double bond shown at a peak of $1610,1590c{m^{ - 1}}$ and the nitro group was shown at $1495,1340c{m^{ - 1}}$.
Therefore, from the above explanation we can say that the spectra in case of ortho is a bit messier than para nitrophenol and the nitro group and carbon-oxygen bonds and alcohol stretches at $1600 \sim 1300c{m^{ - 1}}$, possessing additional splitting and are way stronger than para nitrophenol. In the case of a para molecule, there is symmetry present in the structures.
Note: Always remember that the main difference between ortho and para nitrophenol is due to the symmetry in case of para nitrophenol which is absent in case of ortho nitrophenol which is the reason for weaker peaks of various bonding in para forms.
Complete step-by-step answer:
As we know that the structures of ortho- nitrophenol can be given as shown below:

And the structure of para-nitrophenol is given as shown below:

Now, we also know that infrared spectroscopy results are observed on the basis of the peak seen in graphs provided for the particular molecule. Now the ortho and nitrophenol structures as we can see possess carbon-carbon single bonds and double bonds, carbon and hydrogen single bonds, carbon and oxygen single bonds, oxygen and hydrogen single bond and nitro group.
So, in case of ortho phenol, the carbon-carbon double bonds are observed to peak at \[1610 \sim 1590c{m^{ - 1}}\] which are generally medium to weak and sharp in appearance. Similarly, the carbon-hydrogen bonds are shown at peak at $3445,3240c{m^{ - 1}}$ respectively which are medium to weak and sharp in appearance.
And the carbon and oxygen bonds are shown at a peak of about $1300 \sim 1000c{m^{ - 1}}$ and these are strong and sharp in appearance whereas the alcoholic bonds are shown at $3650 \sim 3200c{m^{ - 1}}$ and are medium to strong and broad in appearance. Lastly, the nitro group which is shown at $1570,1310c{m^{ - 1}}$ and is strong and sharp in appearance.
And in case of para nitrophenol, the alcoholic bonds are shown as superimposed by carbon-hydrogen bonds at a peak of about $3365c{m^{ - 1}}$, the carbon-carbon double bond shown at a peak of $1610,1590c{m^{ - 1}}$ and the nitro group was shown at $1495,1340c{m^{ - 1}}$.
Therefore, from the above explanation we can say that the spectra in case of ortho is a bit messier than para nitrophenol and the nitro group and carbon-oxygen bonds and alcohol stretches at $1600 \sim 1300c{m^{ - 1}}$, possessing additional splitting and are way stronger than para nitrophenol. In the case of a para molecule, there is symmetry present in the structures.
Note: Always remember that the main difference between ortho and para nitrophenol is due to the symmetry in case of para nitrophenol which is absent in case of ortho nitrophenol which is the reason for weaker peaks of various bonding in para forms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




