
What does an octahedral geometry look like?
Answer
493.8k+ views
Hint: Geometry of any compound is decided by the spatial arrangement of electronegative groups or ligands around the central atom. The number of groups, the presence or absence of lone pairs, the repulsion between bond pairs and the allowed angles together decide the geometry of a compound.
Complete answer:
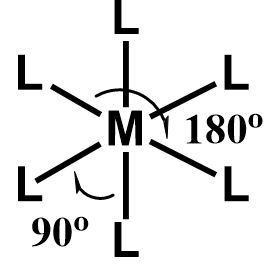
An octahedral geometry derives its name from a regular octahedron. The atoms, molecules or groups are placed in such a manner that they appear to form the vertices of a regular octahedron.
This geometry is observed only when there are six ligands or electronegative groups attached to the central element. It is an extremely symmetrical geometry in which four ligands or groups are placed in a square planar fashion where the ligands are in the same plane as that of the central atom. The remaining two ligands are placed anti-parallel to each other in an axial fashion, lying perpendicular to the ligands placed in the plane.
All the bond distances are equal in an octahedral geometry where all the six positions are equivalent and there is no difference between the equatorial and axial positions.
The six pairs or ligands are placed perpendicular to each other and only the vertically opposite groups are placed in a linear fashion making an angle of \[{180^ \circ }\] .
\[ \Rightarrow \] Thus, an octahedral geometry looks like this:
Note:
Octahedral geometry can be formed by elements of the main group as well as transition metals. When the axial position of an octahedral geometry is replaced by lone pairs, then it is called a square planar geometry as lone pairs are not a direct part of geometry.
Complete answer:
An octahedral geometry derives its name from a regular octahedron. The atoms, molecules or groups are placed in such a manner that they appear to form the vertices of a regular octahedron.
This geometry is observed only when there are six ligands or electronegative groups attached to the central element. It is an extremely symmetrical geometry in which four ligands or groups are placed in a square planar fashion where the ligands are in the same plane as that of the central atom. The remaining two ligands are placed anti-parallel to each other in an axial fashion, lying perpendicular to the ligands placed in the plane.
All the bond distances are equal in an octahedral geometry where all the six positions are equivalent and there is no difference between the equatorial and axial positions.
The six pairs or ligands are placed perpendicular to each other and only the vertically opposite groups are placed in a linear fashion making an angle of \[{180^ \circ }\] .
\[ \Rightarrow \] Thus, an octahedral geometry looks like this:

Note:
Octahedral geometry can be formed by elements of the main group as well as transition metals. When the axial position of an octahedral geometry is replaced by lone pairs, then it is called a square planar geometry as lone pairs are not a direct part of geometry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




