
How does borane exist in THF solution?
A.As the trimer \[{{\text{B}}_{\text{3}}}{{\text{H}}_{\text{9}}}\]
B.As the complex between borane and THF
C.As the dimer \[{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}\]
D.As the monomer \[{\text{B}}{{\text{H}}_{\text{3}}}\]
Answer
571.5k+ views
Hint:
The borane has the molecular formula,\[{\text{B}}{{\text{H}}_{\text{3}}}\] and it always exist in the dimer form \[{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}\] known as diborane. They mostly form complex ions in reaction with THF.
Complete step by step answer:
Borane exists in tetrahydrofuran (THF) solution as a complex with dipolar bond between borane and THF. The resulting complex can be used for reduction and hydroboration reactions. This solution has wide application in synthesis of various organic compounds.
One of the common reductions of carboxylic acids to alcohols can be done by \[{\text{B}}{{\text{H}}_{\text{3}}} - {\text{THF}}\] complex. This complex solution can be used to reduce even amino acids to amino alcohols. Commercially, it is available in a concentration of one mole per litre packed in volumes ranging from 25 to 800 mL.
It is much more convenient to work with the solution than with a gas. Even so, the solution must be stored at 2 to 8 °C, and it must have a stabilizer added.
The equilibrium reaction between tetrahydrofuran (THF) and borane can be represented as below:
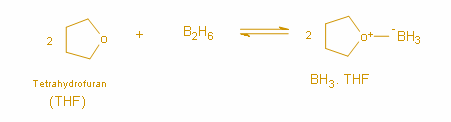
Thus, the equilibrium reaction clearly depicts that Borane exists in the THF solution as the complex between borane and THF.
So, the correct option is B.
Note:
Boranes exist in both neutral and ionic form. Based on the structural features, they can be mainly classified into three classes listed below:
Closo boranes,
Nido boranes and
Arachno boranes.
The borane has the molecular formula,\[{\text{B}}{{\text{H}}_{\text{3}}}\] and it always exist in the dimer form \[{{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}\] known as diborane. They mostly form complex ions in reaction with THF.
Complete step by step answer:
Borane exists in tetrahydrofuran (THF) solution as a complex with dipolar bond between borane and THF. The resulting complex can be used for reduction and hydroboration reactions. This solution has wide application in synthesis of various organic compounds.
One of the common reductions of carboxylic acids to alcohols can be done by \[{\text{B}}{{\text{H}}_{\text{3}}} - {\text{THF}}\] complex. This complex solution can be used to reduce even amino acids to amino alcohols. Commercially, it is available in a concentration of one mole per litre packed in volumes ranging from 25 to 800 mL.
It is much more convenient to work with the solution than with a gas. Even so, the solution must be stored at 2 to 8 °C, and it must have a stabilizer added.
The equilibrium reaction between tetrahydrofuran (THF) and borane can be represented as below:

Thus, the equilibrium reaction clearly depicts that Borane exists in the THF solution as the complex between borane and THF.
So, the correct option is B.
Note:
Boranes exist in both neutral and ionic form. Based on the structural features, they can be mainly classified into three classes listed below:
Closo boranes,
Nido boranes and
Arachno boranes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




