
How does crystal field theory explain high spin and low spin states of complexes?
Answer
515.1k+ views
Hint: Crystal field theory explains the concept behind bonding interactions between ligands and transition metal atoms. It demonstrates the effect of attraction between positively charged metal cations and non-bonding electrons of the ligand. Degeneracy of d orbitals is observed in CFT.
Complete answer:
According to crystal field theory, the electrons which are present in the d orbital of the transition metal atom repel the electrons of ligands. Therefore, it is observed that the d-orbitals closer to the ligands will consist of higher energy as compared to those which are far away from the ligands. Thus, it results in the splitting of energy of d orbitals. The splitting of d orbitals depends on the following factors:
The nature of the central metal ion.
Oxidation state of central metal ion (higher the oxidation state, the greater will be the splitting of d orbital)
The way of arrangement of ligands around the central metal ion.
The nature of the ligand present.
The splitting of d-orbitals is different for basic structure of complexes as shown below:
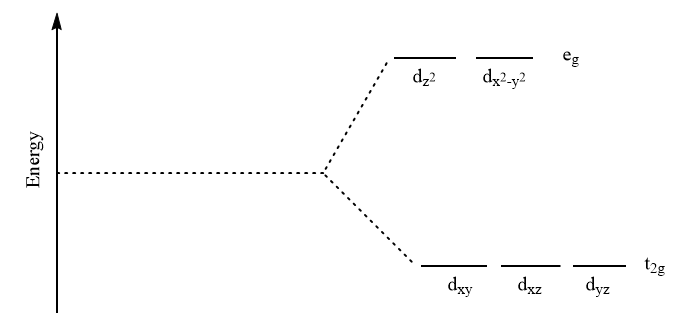
For octahedral complexes, the splitting of d-orbital takes place as follows:
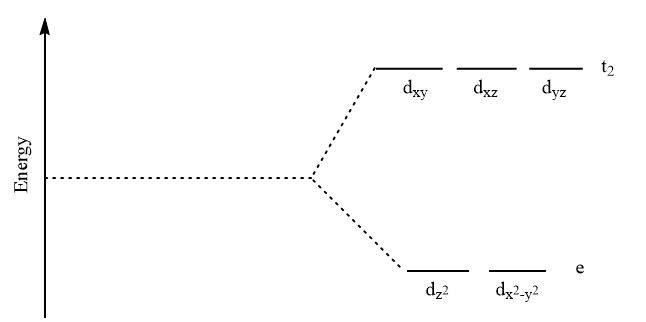
For tetrahedral complexes, the splitting of d-orbital takes place as follows:
Ligands which are responsible for large splitting of d orbitals are known as strong field ligands. For example, $ C{{N}^{-}} $ and $ CO $ . It is a very unfavourable condition to allocate electrons in higher energy orbitals for the complexes which form dative bonds with these ligands. Therefore, pairing of electrons is first completed in d orbitals with lower energy and thus, these complexes are known as low spin complexes.
On the other hand, the ligands like $ {{I}^{-}} $
and $ B{{r}^{-}} $ are considered to be weak field ligands because they cause small splitting of d orbitals. Therefore, for this case it is much easier to allocate electrons to higher energy d -orbital. Hence, no pairing of electrons takes place and such complexes are known as high spin complexes.
Note:
After degeneracy or splitting of d orbitals, the difference in the energy of orbitals is known as crystal field stabilization energy. In simple words, it is the amount of energy through which the complex stabilizes on degenerating its d orbital.
Complete answer:
According to crystal field theory, the electrons which are present in the d orbital of the transition metal atom repel the electrons of ligands. Therefore, it is observed that the d-orbitals closer to the ligands will consist of higher energy as compared to those which are far away from the ligands. Thus, it results in the splitting of energy of d orbitals. The splitting of d orbitals depends on the following factors:
The nature of the central metal ion.
Oxidation state of central metal ion (higher the oxidation state, the greater will be the splitting of d orbital)
The way of arrangement of ligands around the central metal ion.
The nature of the ligand present.
The splitting of d-orbitals is different for basic structure of complexes as shown below:
For octahedral complexes, the splitting of d-orbital takes place as follows:

For tetrahedral complexes, the splitting of d-orbital takes place as follows:

Ligands which are responsible for large splitting of d orbitals are known as strong field ligands. For example, $ C{{N}^{-}} $ and $ CO $ . It is a very unfavourable condition to allocate electrons in higher energy orbitals for the complexes which form dative bonds with these ligands. Therefore, pairing of electrons is first completed in d orbitals with lower energy and thus, these complexes are known as low spin complexes.
On the other hand, the ligands like $ {{I}^{-}} $
and $ B{{r}^{-}} $ are considered to be weak field ligands because they cause small splitting of d orbitals. Therefore, for this case it is much easier to allocate electrons to higher energy d -orbital. Hence, no pairing of electrons takes place and such complexes are known as high spin complexes.
Note:
After degeneracy or splitting of d orbitals, the difference in the energy of orbitals is known as crystal field stabilization energy. In simple words, it is the amount of energy through which the complex stabilizes on degenerating its d orbital.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




