
How does oxygen change with altitude?
Answer
558.6k+ views
Hint: The pressure exerted by an individual gas in a mixture is known as its partial pressure. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture which is known as Dalton’s Law.
Complete answer:
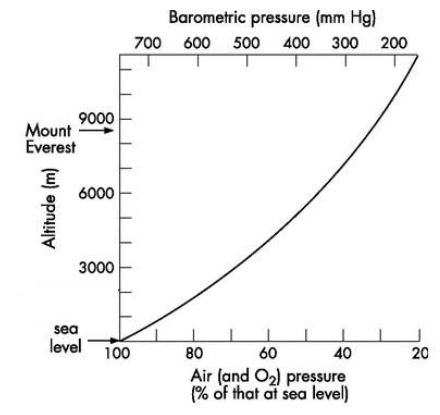
At sea-level, the air is denser and is more compressed. As we move upwards, the air becomes less compressed and is therefore thinner. The composition of oxygen in air is around 21%. As the altitude increases, the composition of oxygen doesn’t change, but the partial pressure of oxygen starts to decrease. The atmospheric pressure at sea-level is 760mm Hg. It falls to about 380mm Hg at 5500 m and only 230mm Hg at 8900 m.
At sea-level, the partial pressure of oxygen is 159mm Hg, whereas at the summit of Mount Everest, the partial pressure of oxygen is only 53mm Hg. This means that at higher altitudes, oxygen molecules are further apart to each other, i.e. there are fewer molecules of oxygen in the same volume of air as we inhale. This is referred to as ‘hypoxia’.
At higher altitudes, the breathing rate increases so as to increase the oxygen uptake. But still then, less oxygen reaches to the muscles which limit exercise performance. At the initial hours of altitude exposure, water loss also increases resulting in dehydration. It also increases our metabolism while suppressing our appetite. People start to get adjusted to the lower oxygen environment when exposed to altitude for several weeks. This is known as acclimation. Despite the adaptations in the body to compensate for hypoxic conditions physical performance remains worse as compared to sea-level.
Note: If the people don’t acclimatize properly, there is a great chance of developing altitude sickness. The altitude sickness can be of three kinds:
-Acute Mountain Sickness (AMS) is the mildest form and is very common.
-High Altitude Pulmonary Edema (HAPE) is a buildup of fluid in the lungs that can be very dangerous and even life threatening.
-High Altitude Cerebral Edema (HACE) is the most severe form of high altitude sickness. There is a buildup of fluid in the brain which is life threatening and needs medical attention right away.
Complete answer:
At sea-level, the air is denser and is more compressed. As we move upwards, the air becomes less compressed and is therefore thinner. The composition of oxygen in air is around 21%. As the altitude increases, the composition of oxygen doesn’t change, but the partial pressure of oxygen starts to decrease. The atmospheric pressure at sea-level is 760mm Hg. It falls to about 380mm Hg at 5500 m and only 230mm Hg at 8900 m.
At sea-level, the partial pressure of oxygen is 159mm Hg, whereas at the summit of Mount Everest, the partial pressure of oxygen is only 53mm Hg. This means that at higher altitudes, oxygen molecules are further apart to each other, i.e. there are fewer molecules of oxygen in the same volume of air as we inhale. This is referred to as ‘hypoxia’.
At higher altitudes, the breathing rate increases so as to increase the oxygen uptake. But still then, less oxygen reaches to the muscles which limit exercise performance. At the initial hours of altitude exposure, water loss also increases resulting in dehydration. It also increases our metabolism while suppressing our appetite. People start to get adjusted to the lower oxygen environment when exposed to altitude for several weeks. This is known as acclimation. Despite the adaptations in the body to compensate for hypoxic conditions physical performance remains worse as compared to sea-level.

Note: If the people don’t acclimatize properly, there is a great chance of developing altitude sickness. The altitude sickness can be of three kinds:
-Acute Mountain Sickness (AMS) is the mildest form and is very common.
-High Altitude Pulmonary Edema (HAPE) is a buildup of fluid in the lungs that can be very dangerous and even life threatening.
-High Altitude Cerebral Edema (HACE) is the most severe form of high altitude sickness. There is a buildup of fluid in the brain which is life threatening and needs medical attention right away.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




