
Why does p-dichlorobenzene have a higher melting point than its o- and m-isomers?
Answer
592.8k+ views
Hint: When molecules fit perfectly and closely in a crystal lattice, the packing efficiency increases, and when molecules are closely fit in a lattice, the number of molecules and energy of crystal lattice both increases.
Complete answer:
Molecular symmetry has an immense effect on the melting properties and solubility of organic compounds. As a rule, symmetrical molecules in crystalline form are known to have higher melting points and show lower solubilities compared with molecules of similar structure but having lower symmetry. Symmetry in a molecule gives it a positive amount of residual entropy in the solid phase. This means that the entropy of a crystal having symmetric molecules is greater than the entropy of a crystal of a similar , but non-symmetric molecule.
The more symmetrical the compounds are , the easier it will be to accommodate in lattice and the fewer spaces there will be between the molecules. Symmetric structure imparts better packing.
o-dichlorobenzene and m-dichlorobenzene do not possess symmetry , therefore they will not be efficiently packed, however, this is not the case with p-dichlorobenzene.
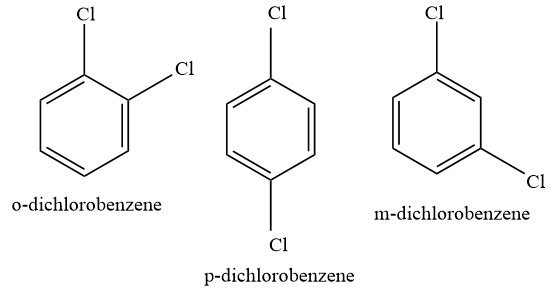
p-dichlorobenzene exhibits symmetry, which helps it in better packing efficiency. In crystal lattice, para isomer fits more closely as compared to meta and ortho isomer. And because of this reason, more energy is required to break the crystal lattice of para isomers, and lesser energy is required to break the crystal of meta and ortho isomers.
Hence, para-dichlorobenzene has a higher melting point than meta and ortho dichlorobenzene.
Note: Melting point of p-dichlorobenzene is 323K and boiling point is 448K. And in comparison to that, the melting point of meta-dichlorobenzene is 249K boiling point is 446K and , melting point of ortho-dichlorobenzene is 256K boiling point is 443K. You will notice that boiling points do not have such variations.
Complete answer:
Molecular symmetry has an immense effect on the melting properties and solubility of organic compounds. As a rule, symmetrical molecules in crystalline form are known to have higher melting points and show lower solubilities compared with molecules of similar structure but having lower symmetry. Symmetry in a molecule gives it a positive amount of residual entropy in the solid phase. This means that the entropy of a crystal having symmetric molecules is greater than the entropy of a crystal of a similar , but non-symmetric molecule.
The more symmetrical the compounds are , the easier it will be to accommodate in lattice and the fewer spaces there will be between the molecules. Symmetric structure imparts better packing.
o-dichlorobenzene and m-dichlorobenzene do not possess symmetry , therefore they will not be efficiently packed, however, this is not the case with p-dichlorobenzene.

p-dichlorobenzene exhibits symmetry, which helps it in better packing efficiency. In crystal lattice, para isomer fits more closely as compared to meta and ortho isomer. And because of this reason, more energy is required to break the crystal lattice of para isomers, and lesser energy is required to break the crystal of meta and ortho isomers.
Hence, para-dichlorobenzene has a higher melting point than meta and ortho dichlorobenzene.
Note: Melting point of p-dichlorobenzene is 323K and boiling point is 448K. And in comparison to that, the melting point of meta-dichlorobenzene is 249K boiling point is 446K and , melting point of ortho-dichlorobenzene is 256K boiling point is 443K. You will notice that boiling points do not have such variations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




