
Does propanone show tautomerism?
Answer
542.7k+ views
Hint: The tautomers can be identified as structural isomers of each other, or in other words tautomers are the compounds which differ from each other on the basis of position of the protons and electrons in the compound.
Complete step by step answer:
We need to know the definitions of tautomerism or isomerism in general before answering this question. Isomers are compounds which have the same molecular formula but have different structural formulas. Now, this isomerism can be majorly divided into two groups which are, structural isomers and stereoisomers. Among the category of structural isomers there are six subcategories which are chain isomers, position isomers, functional isomers, metamers, tautomers and ring chain. Whereas, in the case of stereoisomers, there are only two subcategories which are geometrical isomers and optical isomers.
Now, as we can see the basics are cleared, now we would discuss tautomerism as it is a subcategory of structural isomers.
Tautomerism is a phenomenon in which one chemical compound or a molecule tends to exist in more than one interconvertible form. One type of tautomerism is keto-enol tautomerism. This consists of two interconvertible structures of the same compound, one of which has ketone group and other has enol group or unsaturated alcoholic group.
Propanone shows keto-enol tautomerism, which is shown below.
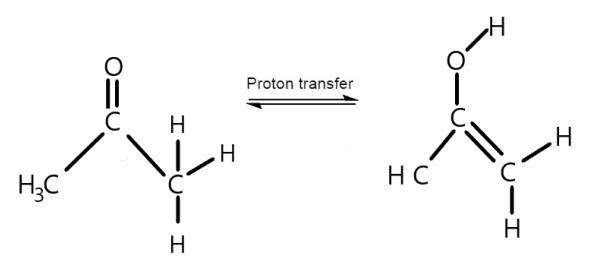
So, the answer is yes.
Note: The isomerism is divided into two major categories namely structural and stereoisomers. The structural isomers deal with the position of the carbon and the functional groups and it is divided into six subcategories accordingly.
The stereoisomerism deals with the spatial arrangements of the functional groups and is divided into two subcategories accordingly. Keto-enol tautomerism is a type of structural isomerism.
Complete step by step answer:
We need to know the definitions of tautomerism or isomerism in general before answering this question. Isomers are compounds which have the same molecular formula but have different structural formulas. Now, this isomerism can be majorly divided into two groups which are, structural isomers and stereoisomers. Among the category of structural isomers there are six subcategories which are chain isomers, position isomers, functional isomers, metamers, tautomers and ring chain. Whereas, in the case of stereoisomers, there are only two subcategories which are geometrical isomers and optical isomers.
Now, as we can see the basics are cleared, now we would discuss tautomerism as it is a subcategory of structural isomers.
Tautomerism is a phenomenon in which one chemical compound or a molecule tends to exist in more than one interconvertible form. One type of tautomerism is keto-enol tautomerism. This consists of two interconvertible structures of the same compound, one of which has ketone group and other has enol group or unsaturated alcoholic group.
Propanone shows keto-enol tautomerism, which is shown below.

So, the answer is yes.
Note: The isomerism is divided into two major categories namely structural and stereoisomers. The structural isomers deal with the position of the carbon and the functional groups and it is divided into six subcategories accordingly.
The stereoisomerism deals with the spatial arrangements of the functional groups and is divided into two subcategories accordingly. Keto-enol tautomerism is a type of structural isomerism.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




