
Why does sodium chloride on heating with sodium vapours acquire a yellow colour?
Answer
590.1k+ views
Hint: Schottky defects are caused when anions are replaced by electrons in their lattice sites.
Complete step by step answer:
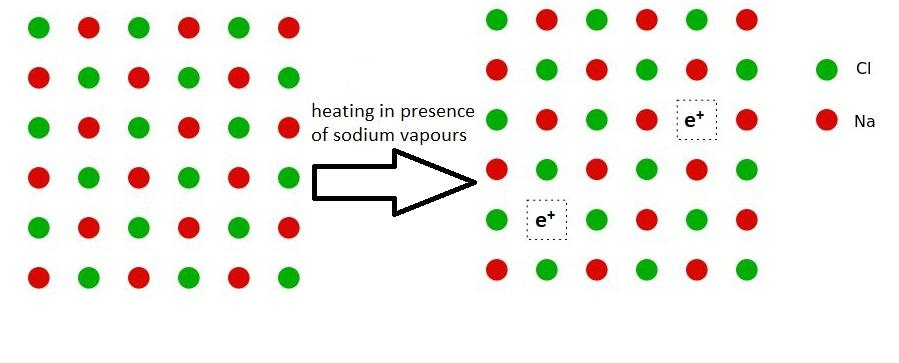
When sodium chloride crystals are heated in an atmosphere of sodium vapours, the chloride ions leave their lattice sites. These vacant spaces are then filled by electrons. Electrons move to the lattice sites so that the crystal remains electrically neutral. This type of defect is observed in the crystals which are likely to form Schottky Defects.
For example, Alkali metal halides like NaCl and KCl show this Schottky defect. When crystals of NaCl are heated in an atmosphere of sodium vapour, the sodium atoms get deposited on the surface of the crystal. The chloride ions, then, diffuse to the surface of the crystal and combine with the sodium atoms to form sodium chloride. This happens when sodium atoms lose electrons. This electron coming from the sodium atom will go to the empty lattice site that was left by the chloride anions. As a result, the crystals now have an excess of sodium. The anionic sites that are occupied by unpaired electrons are called F – centres. These F – centres impart yellow colour to the crystals of sodium chloride. The colour is a result of the excitation of these electrons due to absorption of energy from the visible spectrum of light that is falling on the crystals.
Here is the diagrammatic representation of the same phenomenon –
Note:
Similarly, excess lithium vapours in the atmosphere while lithium chloride crystals are heated turns the lithium chloride crystals pink and excess of potassium turns the potassium chloride crystals violet (or lilac).
Metal excess defects can also be caused due to presence of extra cations at the interstitial sites. This happens in the case of Zinc oxide.
Complete step by step answer:
When sodium chloride crystals are heated in an atmosphere of sodium vapours, the chloride ions leave their lattice sites. These vacant spaces are then filled by electrons. Electrons move to the lattice sites so that the crystal remains electrically neutral. This type of defect is observed in the crystals which are likely to form Schottky Defects.
For example, Alkali metal halides like NaCl and KCl show this Schottky defect. When crystals of NaCl are heated in an atmosphere of sodium vapour, the sodium atoms get deposited on the surface of the crystal. The chloride ions, then, diffuse to the surface of the crystal and combine with the sodium atoms to form sodium chloride. This happens when sodium atoms lose electrons. This electron coming from the sodium atom will go to the empty lattice site that was left by the chloride anions. As a result, the crystals now have an excess of sodium. The anionic sites that are occupied by unpaired electrons are called F – centres. These F – centres impart yellow colour to the crystals of sodium chloride. The colour is a result of the excitation of these electrons due to absorption of energy from the visible spectrum of light that is falling on the crystals.
Here is the diagrammatic representation of the same phenomenon –

Note:
Similarly, excess lithium vapours in the atmosphere while lithium chloride crystals are heated turns the lithium chloride crystals pink and excess of potassium turns the potassium chloride crystals violet (or lilac).
Metal excess defects can also be caused due to presence of extra cations at the interstitial sites. This happens in the case of Zinc oxide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




