
Draw a diagram for electroplating an article with silver.
Answer
579.6k+ views
Hint: Silver is a metal with symbol Ag with atomic number 47. It is found in earth’s crust in pure and free form. Electroplating is the process of putting a metal coating on an article by the means of a direct electric current through the reduction of cations of that metal.
Complete answer
The portion that is to be coated in electroplating acts as the cathode i.e. negative electrode. The solution of the salt of the metal to be coated acts as the electrolyte. In this electrolytic cell, either the block of the metal to be coated or any inert conductive material is used as the anode i.e. positive electrode. Electroplating has various utilities in various industries. Generally, electroplating is used to improve the surface qualities like resistance to corrosion, lubricity, reflectivity, electrical conductivity, appearance, etc. The diagram for electroplating an article with silver is shown below.
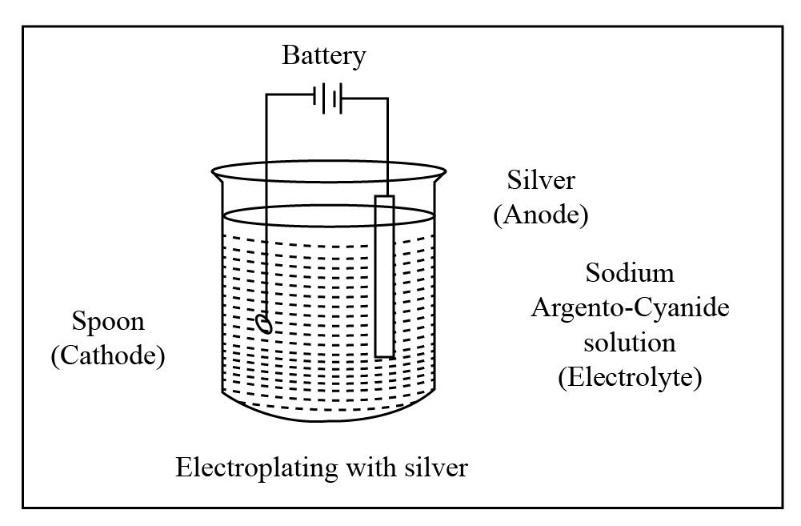
For electroplating an article with silver, we have to construct an electrolytic cell because the electroplating is done by the means of direct electric current. So for the flow of direct electric current the construction of electrolytic cell is necessary. To perform the process of electroplating an article with silver let us take an example of an article say spoon.
Now, in the required electrolytic cell the article to be coated i.e. the spoon will act as the cathode. Silver i.e. the metal to be coated will act as anode i.e. the positive electrode. Now, for the conduction of electric current we have to make a suitable choice of electrolyte which is sodium argento-cyanide solution.
Note:
Thus, the process of electroplating of an article is carried out with the means of direct current through the electrolyte cell. The article to be coated acts as the cathode, whereas the silver acts as anode. The electrolyte solution used is a salt solution named sodium argento-cyanide solution.
Complete answer
The portion that is to be coated in electroplating acts as the cathode i.e. negative electrode. The solution of the salt of the metal to be coated acts as the electrolyte. In this electrolytic cell, either the block of the metal to be coated or any inert conductive material is used as the anode i.e. positive electrode. Electroplating has various utilities in various industries. Generally, electroplating is used to improve the surface qualities like resistance to corrosion, lubricity, reflectivity, electrical conductivity, appearance, etc. The diagram for electroplating an article with silver is shown below.

For electroplating an article with silver, we have to construct an electrolytic cell because the electroplating is done by the means of direct electric current. So for the flow of direct electric current the construction of electrolytic cell is necessary. To perform the process of electroplating an article with silver let us take an example of an article say spoon.
Now, in the required electrolytic cell the article to be coated i.e. the spoon will act as the cathode. Silver i.e. the metal to be coated will act as anode i.e. the positive electrode. Now, for the conduction of electric current we have to make a suitable choice of electrolyte which is sodium argento-cyanide solution.
Note:
Thus, the process of electroplating of an article is carried out with the means of direct current through the electrolyte cell. The article to be coated acts as the cathode, whereas the silver acts as anode. The electrolyte solution used is a salt solution named sodium argento-cyanide solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




