
Draw a labelled diagram of ‘Standard Hydrogen Electrode’.
Answer
585k+ views
Hint:SHE consists of platinum electrode coated in platinum black and dipped in \[{\text{1 M}}\] solution of \[{\text{HCl}}\] acid. Also the pure hydrogen gas is bubbled through the solution.
SHE is a type of gas electrode which acts as an indicator electrode for the determination of \[{\text{pH}}\] value. Depending upon the nature of half-cell, SHE used as either an anode or cathode.
Complete step by step answer:
The labelled diagram for the Standard Hydrogen Electrode is given below:
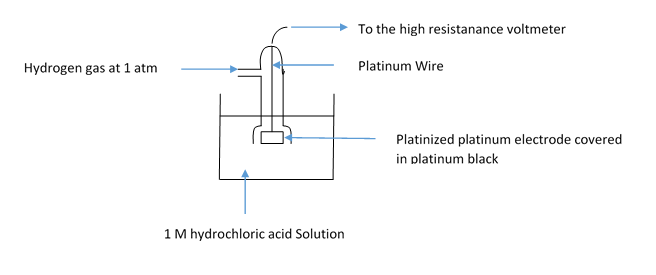
Let’s start with discussing the basic working of the Standard Hydrogen Electrode (SHE). SHE consists of platinum electrode coated in platinum black and dipped in \[{\text{1 M}}\] solution of \[{\text{HCl}}\] acid. Also the pure hydrogen gas is bubbled through the solution. The concentration of both the oxidizing and reducing hydrogen are kept at unity. The SHE works at \[{0^o}C\] temperature and 1 bar pressure which is the STP condition.
The Standard Hydrogen electrode is a redox electrode and both the oxidation and reduction happens in it. The redox happens with hydrogen only and both are kept at unity. There are also Normal Hydrogen Electrode and reverse hydrogen electrode which are little different from SHE.
Note:
The standard hydrogen electrode (SHE) is used as the universal reference for reporting a half-cell potential. It has been used as a referral electrode for many studies and also as an indicating electrode for determining the pH of the solution. It is to be noted that the assembly of SHE is not easy to assemble.
SHE is a type of gas electrode which acts as an indicator electrode for the determination of \[{\text{pH}}\] value. Depending upon the nature of half-cell, SHE used as either an anode or cathode.
Complete step by step answer:
The labelled diagram for the Standard Hydrogen Electrode is given below:

Let’s start with discussing the basic working of the Standard Hydrogen Electrode (SHE). SHE consists of platinum electrode coated in platinum black and dipped in \[{\text{1 M}}\] solution of \[{\text{HCl}}\] acid. Also the pure hydrogen gas is bubbled through the solution. The concentration of both the oxidizing and reducing hydrogen are kept at unity. The SHE works at \[{0^o}C\] temperature and 1 bar pressure which is the STP condition.
The Standard Hydrogen electrode is a redox electrode and both the oxidation and reduction happens in it. The redox happens with hydrogen only and both are kept at unity. There are also Normal Hydrogen Electrode and reverse hydrogen electrode which are little different from SHE.
Note:
The standard hydrogen electrode (SHE) is used as the universal reference for reporting a half-cell potential. It has been used as a referral electrode for many studies and also as an indicating electrode for determining the pH of the solution. It is to be noted that the assembly of SHE is not easy to assemble.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




