
Draw a neat and labelled diagram of a Bessemer converter used in the extraction of copper.
Answer
545.7k+ views
Hint: The Bessemer cycle was the primary reasonable modern cycle for the large scale manufacturing of steel from liquid pig iron before the improvement of the open hearth heater. The key guideline is expulsion of pollutants from the iron by oxidation with air being blown through the liquid iron. The oxidation likewise raises the temperature of the iron mass and keeps it liquid.
Complete step by step answer:
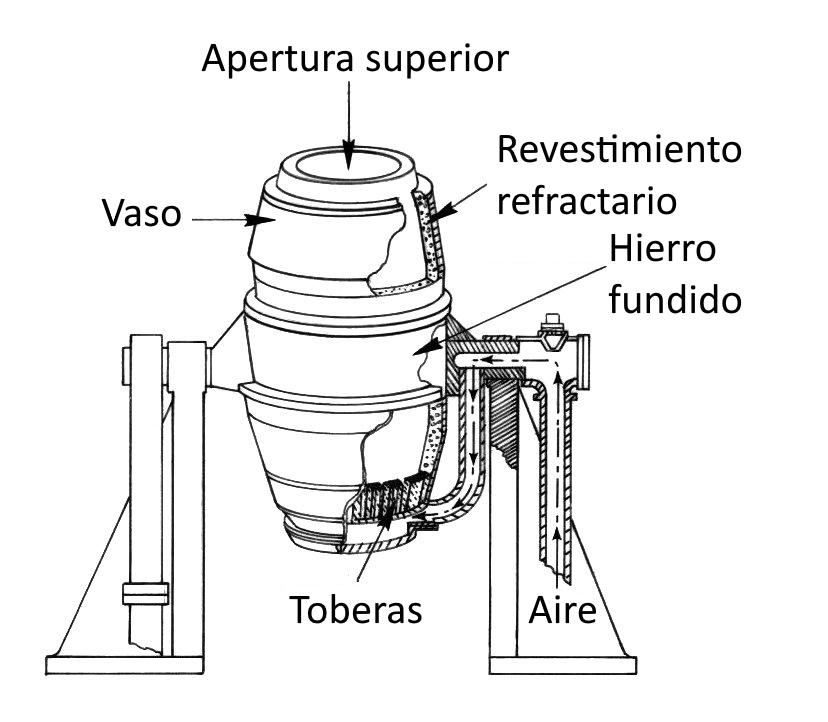
Copper metal is take out from molten matte concluded bessemerisation. The matte is introduced into bessemer converter which uphold by tuyeres. The air is blown through the molten matte. Explosion of air converts \[C{u_{2}}S\;\] partly into \[C{u_{2}}O\;\] which reacts with residual \[C{u_{2}}S\;\] to give molten copper. \[2C{u_{2}}S + 3{O_2} \to 2C{u_{2}}O + 2S{O_{2}}\] \[2C{u_2}O + C{u_2}S \to 6Cu+ S{O_2}\] The copper so acquired is called "Blister copper" because as it solidifies, \[S{O_2}\;\] concealed in it escapes out as bubbles producing blister on its surfaces. The blister copper is \[99\% \]pure.
Additional information:
The Bessemer converter was a machine and encompassing cycle that elaborate the expulsion of pollutants from pig iron (a kind of iron with a high carbon substance) and its change into steel – a material that had verifiably been expensive and tedious to produce. The vital standard behind its activity was the evacuation of debasements, for example, silicon, manganese and carbon through oxidation, turning the weak, to a great extent unusable pig iron into helpful steel.
The oxidation of pollutants happened in a Bessemer converter, an enormous egg-formed holder in which the iron was softened. The strong iron was embedded through an opening at the top and warmed from the base. When the converter had liquefied the pig iron, pressurized air was infused through and across the fluid metal, constraining the undesirable silicates to respond with oxygen and convert into gas and additionally strong oxides .
When the oxidation cycle had occurred, the usable liquid steel could be spilled out from the compartment straightforwardly by tipping it on a focal turn – the holder was suspended off the ground by a couple of enormous swaggers – while the slag could be skimmed off the surface for reuse or removal. The steel was discharged into enormous molds, where it very well may be set into a wide scope of items.
Note: The Bessemer process altered steel manufacture by decreasing its cost, from pounds40 per long ton to pounds6-7per long ton, along with greatly increasing the gauge and speed of production of this vital raw material. The procedure also decreased the labor supplies for steel-making.
Complete step by step answer:
Copper metal is take out from molten matte concluded bessemerisation. The matte is introduced into bessemer converter which uphold by tuyeres. The air is blown through the molten matte. Explosion of air converts \[C{u_{2}}S\;\] partly into \[C{u_{2}}O\;\] which reacts with residual \[C{u_{2}}S\;\] to give molten copper. \[2C{u_{2}}S + 3{O_2} \to 2C{u_{2}}O + 2S{O_{2}}\] \[2C{u_2}O + C{u_2}S \to 6Cu+ S{O_2}\] The copper so acquired is called "Blister copper" because as it solidifies, \[S{O_2}\;\] concealed in it escapes out as bubbles producing blister on its surfaces. The blister copper is \[99\% \]pure.

Additional information:
The Bessemer converter was a machine and encompassing cycle that elaborate the expulsion of pollutants from pig iron (a kind of iron with a high carbon substance) and its change into steel – a material that had verifiably been expensive and tedious to produce. The vital standard behind its activity was the evacuation of debasements, for example, silicon, manganese and carbon through oxidation, turning the weak, to a great extent unusable pig iron into helpful steel.
The oxidation of pollutants happened in a Bessemer converter, an enormous egg-formed holder in which the iron was softened. The strong iron was embedded through an opening at the top and warmed from the base. When the converter had liquefied the pig iron, pressurized air was infused through and across the fluid metal, constraining the undesirable silicates to respond with oxygen and convert into gas and additionally strong oxides .
When the oxidation cycle had occurred, the usable liquid steel could be spilled out from the compartment straightforwardly by tipping it on a focal turn – the holder was suspended off the ground by a couple of enormous swaggers – while the slag could be skimmed off the surface for reuse or removal. The steel was discharged into enormous molds, where it very well may be set into a wide scope of items.
Note: The Bessemer process altered steel manufacture by decreasing its cost, from pounds40 per long ton to pounds6-7per long ton, along with greatly increasing the gauge and speed of production of this vital raw material. The procedure also decreased the labor supplies for steel-making.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




