
Draw a neat and labelled diagram of $pH$ scale.
Answer
598.8k+ views
Hint : We all are familiar with the term $pH$ and $pH$ scale. $pH$ scale is a scale of acidity and basicity which tells about the acidic and alkaline behaviour of substance .
Complete answer:
> On the $pH$ scale the value ranges from $0$ to $14$. So the more acidic solution will have lower $pH$ and neutral solutions will have $pH$ equal to $7$ and more than $7$ acidic nature decreases and alkaline nature increases .Since $pH$ is defined as the negative of the base $10$ logarithm of the concentration of hydrogen ion ; \[\]$pH$$ = $ $ - {\log _{10}}[{H^ + }]$means $pH$ is a measure of the concentration of ${H^ + }$ ion in the solution.
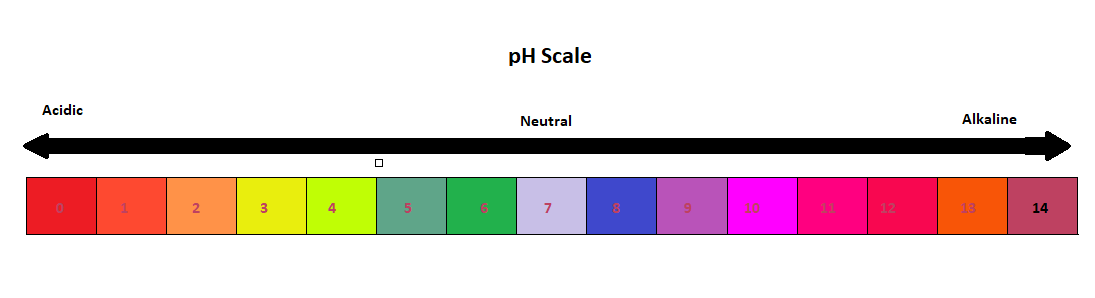 > The $pH$ value can be less than zero which indicates very strong acid and $pH$ value greater than $14$ for a very strong base. A solution where are more ${H^ + }$ ions than $O{H^ - }$ then the solution is acidic and in neutral solution there are equal number of ${H^ + }$ ions and $O{H^ - }$ ions. $pH$ scale has a variety of applications in agriculture, research development, water treatment, environmental monitoring, in quantitative measurement and industrial processing. $pH$ values are affected by temperature so $pH$ applications need to form some temperature compensation to insure standardizes of $pH$ values. We use $pH$ indicators to check the nature of the solution but it does not give precise value of $pH$ because it just changes colour while pH measurement meters used nowadays give an exact value of $pH$.
> The $pH$ value can be less than zero which indicates very strong acid and $pH$ value greater than $14$ for a very strong base. A solution where are more ${H^ + }$ ions than $O{H^ - }$ then the solution is acidic and in neutral solution there are equal number of ${H^ + }$ ions and $O{H^ - }$ ions. $pH$ scale has a variety of applications in agriculture, research development, water treatment, environmental monitoring, in quantitative measurement and industrial processing. $pH$ values are affected by temperature so $pH$ applications need to form some temperature compensation to insure standardizes of $pH$ values. We use $pH$ indicators to check the nature of the solution but it does not give precise value of $pH$ because it just changes colour while pH measurement meters used nowadays give an exact value of $pH$.
Note : Hence $pH$ scale gives information about the acidity and alkalinity of any substance and it ranges from $0$ to $14$ . $pH$ scale is also called the $pH$-acid-base scale. Solutions which are neither acidic nor basic are $7$. So $pH$ is a measure of the hydrogen ion activity in aqueous solution.
Complete answer:
> On the $pH$ scale the value ranges from $0$ to $14$. So the more acidic solution will have lower $pH$ and neutral solutions will have $pH$ equal to $7$ and more than $7$ acidic nature decreases and alkaline nature increases .Since $pH$ is defined as the negative of the base $10$ logarithm of the concentration of hydrogen ion ; \[\]$pH$$ = $ $ - {\log _{10}}[{H^ + }]$means $pH$ is a measure of the concentration of ${H^ + }$ ion in the solution.

Note : Hence $pH$ scale gives information about the acidity and alkalinity of any substance and it ranges from $0$ to $14$ . $pH$ scale is also called the $pH$-acid-base scale. Solutions which are neither acidic nor basic are $7$. So $pH$ is a measure of the hydrogen ion activity in aqueous solution.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




