
Draw a sketch of Bohr's model of an atom with three shells.
Answer
504.6k+ views
Hint: Bohr postulated that
-The Electrons in atoms orbit the nucleus in a circular orbit.
-The electrons can only occupy those orbits whose angular momentum is an integral multiple of $ \dfrac{h}{{2\pi }} $ where h is Planck's constant.
$ mvr = \dfrac{{nh}}{{2\pi }} $ n=1, 2, 3…
-Electrons can gain or lose energy by jumping from one allowed orbit to another, absorbing or emitting radiation with a frequency (ν) determined by the energy difference of the levels.
Complete answer:
In Bohr's model of the atom, positively charged nuclei occupy the centre and negatively charged electrons will revolve around the nucleus in 'fixed' energy levels (n= 1,2,3) or orbits named K, L, M.
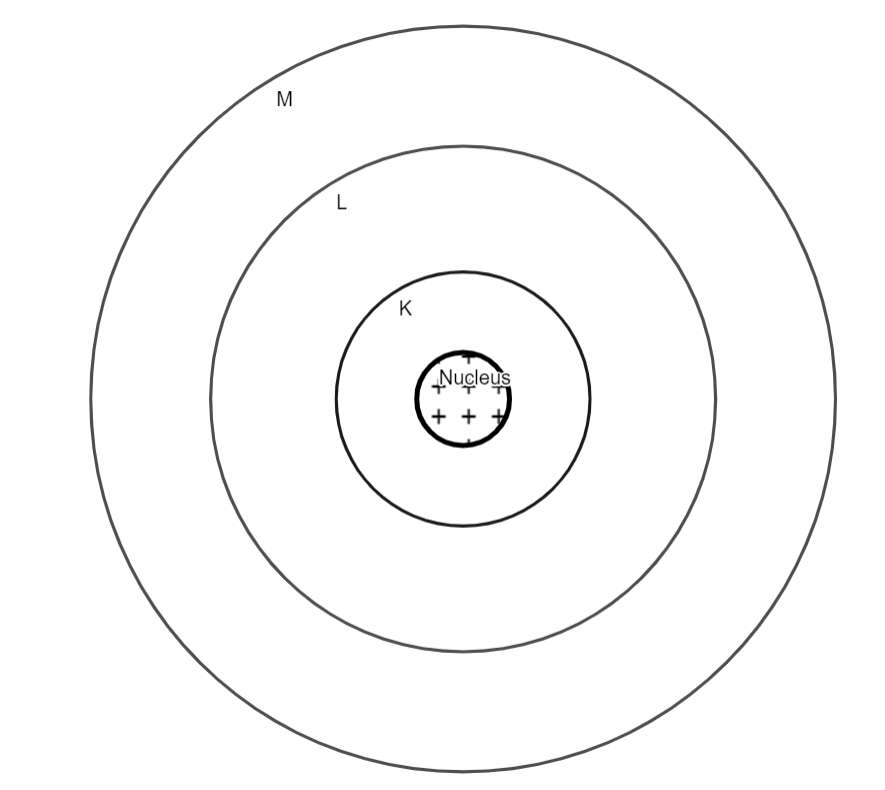
The positively charged nucleus is situated in the centre of the atom. Here the K orbit will contain a maximum of 2 electrons. The L orbit can contain a maximum of 8 electrons whereas the M orbit can contain a maximum of 18 electrons.
This model of atom will account for more stability because the electrons do not lose energy when orbiting and spiral into the nucleus as would happen with the Rutherford model of atoms.
Note:
Bohr’s model of atoms has a lot of drawbacks. It cannot be used to explain the spectra of atoms having more than one electron. Effects observed in the real world experiments such as the Stark effect and Zeeman Effect couldn’t be explained using this model.
Bohr also didn’t account for the wave nature of electrons as suggested by Heisenberg’s Uncertainty principle.
-The Electrons in atoms orbit the nucleus in a circular orbit.
-The electrons can only occupy those orbits whose angular momentum is an integral multiple of $ \dfrac{h}{{2\pi }} $ where h is Planck's constant.
$ mvr = \dfrac{{nh}}{{2\pi }} $ n=1, 2, 3…
-Electrons can gain or lose energy by jumping from one allowed orbit to another, absorbing or emitting radiation with a frequency (ν) determined by the energy difference of the levels.
Complete answer:
In Bohr's model of the atom, positively charged nuclei occupy the centre and negatively charged electrons will revolve around the nucleus in 'fixed' energy levels (n= 1,2,3) or orbits named K, L, M.

The positively charged nucleus is situated in the centre of the atom. Here the K orbit will contain a maximum of 2 electrons. The L orbit can contain a maximum of 8 electrons whereas the M orbit can contain a maximum of 18 electrons.
This model of atom will account for more stability because the electrons do not lose energy when orbiting and spiral into the nucleus as would happen with the Rutherford model of atoms.
Note:
Bohr’s model of atoms has a lot of drawbacks. It cannot be used to explain the spectra of atoms having more than one electron. Effects observed in the real world experiments such as the Stark effect and Zeeman Effect couldn’t be explained using this model.
Bohr also didn’t account for the wave nature of electrons as suggested by Heisenberg’s Uncertainty principle.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




