
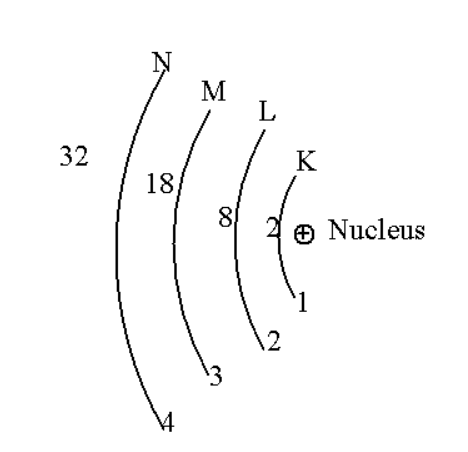
Draw a sketch of the Bohr model of an atom with four shells.
Answer
493.2k+ views
Hint: Neil Bohr explained the stability of atoms with his postulates. The concept of electrons moving in a fixed shell called orbit is given by the Bohr model of atoms. Therefore for sketching the Bohr model for an atom with four shells, we must have to know about the postulates of the Bohr model.
Complete answer:
According to Bohr model of atom we may say that,
a. An atom is made up of particles which are electron, proton and neutron. Electrons are negatively charged particles, while protons are positively charged particles but neutrons have no charge. Since the number of protons and electrons are equal in number for an atom, the charge of an atom is neutral.
b. The protons and neutrons are present inside the nucleus of atoms. Therefore the nucleus is always positively charged.
c. The electrons always revolve around the nucleus in a fixed circular path which is known as shell or energy levels. These energy levels or shells are represented as in order of increasing order, K, L, M, N…….. Or 1, 2, 3, 4……….
d. The electron present in each shell is given the rule of \[2{n^2}\] where n is the shell number. Therefore we can say that the K shell contains only two electrons. The L shell contains four electrons. The M shell contains eighteen electrons. The N shell contains thirty two electrons. This can be depict as,
\[K\left( 2 \right){\text{ ,L}}\left( 8 \right){\text{ ,M}}\left( {18} \right){\text{ ,N}}\left( {32} \right){\text{ }}...............\]
Thus by knowing these postulates we can draw a sketch for atoms consisting of four shells.
Note:
The shells are also known as energy levels. When an electron jumps from higher energy level to lower energy level then it releases energy. When an electron jumps from lower energy level to higher energy level it absorbs energy. We can find the number of electrons in any shell by applying the rule of \[2{n^2}\] given by the Bohr model of atoms.
Complete answer:
According to Bohr model of atom we may say that,
a. An atom is made up of particles which are electron, proton and neutron. Electrons are negatively charged particles, while protons are positively charged particles but neutrons have no charge. Since the number of protons and electrons are equal in number for an atom, the charge of an atom is neutral.
b. The protons and neutrons are present inside the nucleus of atoms. Therefore the nucleus is always positively charged.
c. The electrons always revolve around the nucleus in a fixed circular path which is known as shell or energy levels. These energy levels or shells are represented as in order of increasing order, K, L, M, N…….. Or 1, 2, 3, 4……….
d. The electron present in each shell is given the rule of \[2{n^2}\] where n is the shell number. Therefore we can say that the K shell contains only two electrons. The L shell contains four electrons. The M shell contains eighteen electrons. The N shell contains thirty two electrons. This can be depict as,
\[K\left( 2 \right){\text{ ,L}}\left( 8 \right){\text{ ,M}}\left( {18} \right){\text{ ,N}}\left( {32} \right){\text{ }}...............\]
Thus by knowing these postulates we can draw a sketch for atoms consisting of four shells.

Note:
The shells are also known as energy levels. When an electron jumps from higher energy level to lower energy level then it releases energy. When an electron jumps from lower energy level to higher energy level it absorbs energy. We can find the number of electrons in any shell by applying the rule of \[2{n^2}\] given by the Bohr model of atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




