
Draw all the possible resonance structure for Phenol.
Answer
530.1k+ views
Hint:
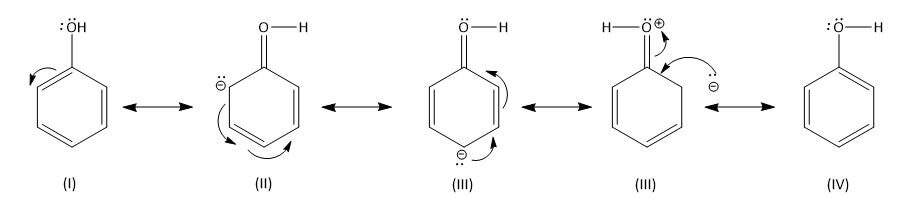
Phenol is the aromatic organic compound. It contains the -\[OH\] group. The oxygen has two electrons which will take part in the resonance. The resonance in the aromatic compound is defined as the delocalization of the electron pair present in the p-orbital of the atom. Phenol contains aromatic rings of benzene with \[OH\] group attached to it, hence it will have more than one resonance structure.
Complete step by step solution
Phenol has a molecular formula as \[{C_6}{H_5}OH\]. The phenomenon of resonance will be shown as -\[OH\] have tendency to donate electron because oxygen have extra pairs of electrons which are non-bonding so, it will participate in the delocalization of the electron the shifting of electron will takes place as aromatic ring have non-bonding electron pair of carbon atom in the p-orbitals. Phenol contains carbon which is \[s{p^2}\] hybridized and attached to the oxygen atom of -\[OH\] group. The oxygen and carbon atom are shorter and the bond length of carbon and hydrogen is larger. The following structure will have electron density different in all resonance structures. At ortho and para position the electron density is maximum, then the meta position. Hence, the electrophile will attract ortho and para positions. Here -\[OH\] is an ortho and para directing group.
Phenol are more acidic than alcohol as in phenol carbon attach to oxygen is \[s{p^2}\] an din alcohol the carbon attach to oxygen is \[s{p^3}\]hybridized due to this the hydrogen will be detached easily from phenol as compare to alcohol.
The resonance structures of phenol are shown below.
Note:
The electron pairs shifting among the atoms of the phenol should be shown with the proper half arrow otherwise it will change the meaning of the structure.
Phenol is the aromatic organic compound. It contains the -\[OH\] group. The oxygen has two electrons which will take part in the resonance. The resonance in the aromatic compound is defined as the delocalization of the electron pair present in the p-orbital of the atom. Phenol contains aromatic rings of benzene with \[OH\] group attached to it, hence it will have more than one resonance structure.
Complete step by step solution
Phenol has a molecular formula as \[{C_6}{H_5}OH\]. The phenomenon of resonance will be shown as -\[OH\] have tendency to donate electron because oxygen have extra pairs of electrons which are non-bonding so, it will participate in the delocalization of the electron the shifting of electron will takes place as aromatic ring have non-bonding electron pair of carbon atom in the p-orbitals. Phenol contains carbon which is \[s{p^2}\] hybridized and attached to the oxygen atom of -\[OH\] group. The oxygen and carbon atom are shorter and the bond length of carbon and hydrogen is larger. The following structure will have electron density different in all resonance structures. At ortho and para position the electron density is maximum, then the meta position. Hence, the electrophile will attract ortho and para positions. Here -\[OH\] is an ortho and para directing group.
Phenol are more acidic than alcohol as in phenol carbon attach to oxygen is \[s{p^2}\] an din alcohol the carbon attach to oxygen is \[s{p^3}\]hybridized due to this the hydrogen will be detached easily from phenol as compare to alcohol.
The resonance structures of phenol are shown below.

Note:
The electron pairs shifting among the atoms of the phenol should be shown with the proper half arrow otherwise it will change the meaning of the structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




