
Draw an electron dot diagram to show the structure of hydronium ions. State the type of bonding present in it.
Answer
562.5k+ views
Hint: Electron dot diagrams are called Lewis dot structures. In this representation, electrons are represented using dots. Bonding in a molecule is presented using the atoms along with their bond pairs and lone pairs.
Complete step by step answer:
Hydronium ion is the common aqueous ion. It is produced by protonation of water molecules. Formation of hydronium ions can be explained using the following electron dot diagram.
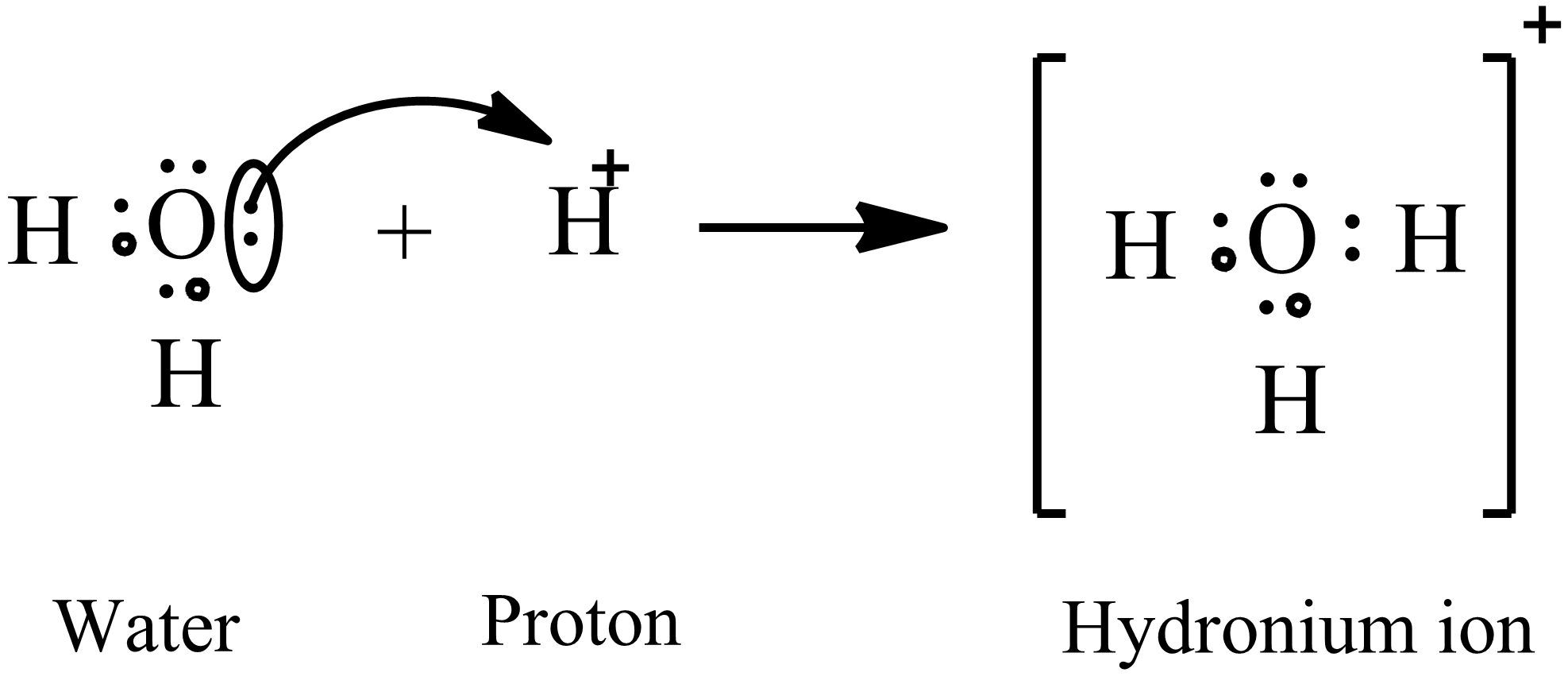
The central atom in water is oxygen. It contains a total six valence electrons. Two of these electrons form bonds with two hydrogen atoms. There are two lone pairs left on the oxygen atom. A proton is a hydrogen cation which does not have any electrons. When a proton approaches the water molecule, the oxygen donates one lone pair towards this proton and forms hydronium ion as shown in the figure. Since both electrons in the formation of hydronium ions are from the oxygen, the bond formed is a coordination bond. The structure of hydronium ion is shown below.
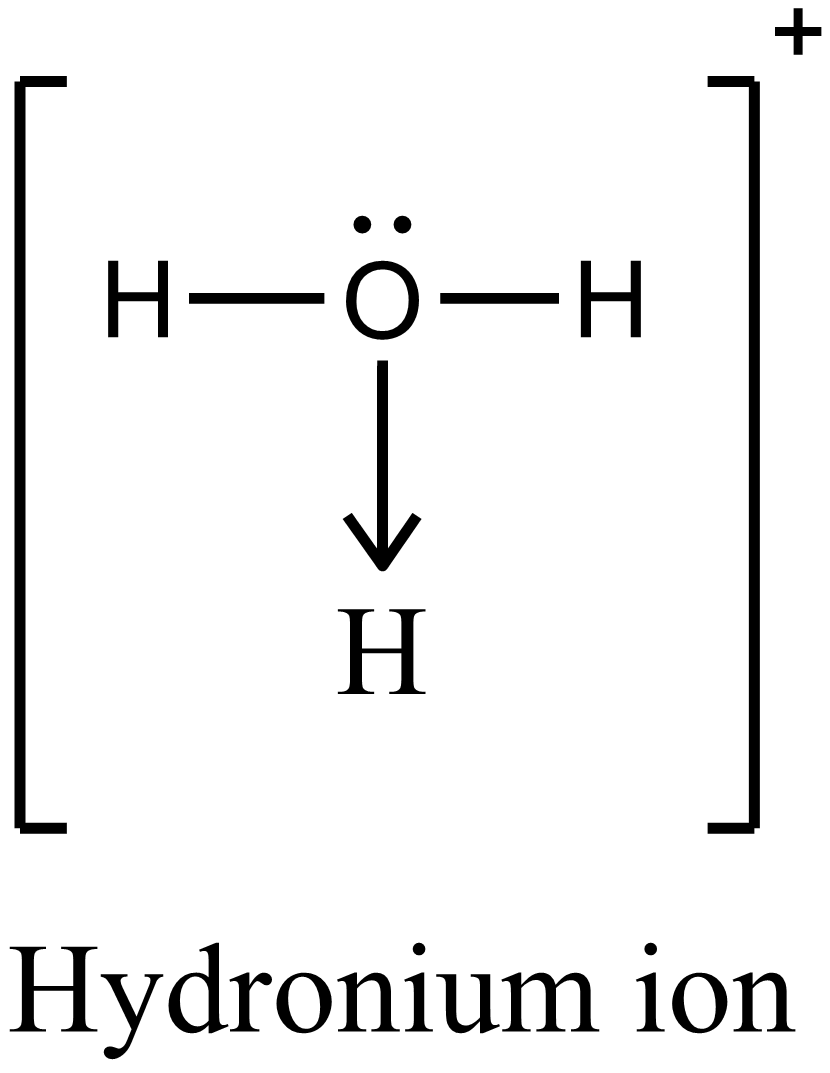
Hence the type of bonding present in hydronium ion is coordination bond.
Additional information-
Structure of hydronium ion is trigonal pyramidal with oxygen in $s{p^3}$ hybridisation. The H-O-H bond angle is ${113^0}$. The one lone pair occupy above the oxygen atom.
Note:
Formula for hydronium ions is ${H_3}{O^ + }$ . According to IUPAC nomenclature hydronium ion is called oxonium ion. Hydronium ions are highly reactive and exist as solvated in water. It exists as a tri-hydrated hydronium ion with chemical formula is ${H_3}{O^ + } \cdot 3{H_2}O$ .
Complete step by step answer:
Hydronium ion is the common aqueous ion. It is produced by protonation of water molecules. Formation of hydronium ions can be explained using the following electron dot diagram.

The central atom in water is oxygen. It contains a total six valence electrons. Two of these electrons form bonds with two hydrogen atoms. There are two lone pairs left on the oxygen atom. A proton is a hydrogen cation which does not have any electrons. When a proton approaches the water molecule, the oxygen donates one lone pair towards this proton and forms hydronium ion as shown in the figure. Since both electrons in the formation of hydronium ions are from the oxygen, the bond formed is a coordination bond. The structure of hydronium ion is shown below.

Hence the type of bonding present in hydronium ion is coordination bond.
Additional information-
Structure of hydronium ion is trigonal pyramidal with oxygen in $s{p^3}$ hybridisation. The H-O-H bond angle is ${113^0}$. The one lone pair occupy above the oxygen atom.
Note:
Formula for hydronium ions is ${H_3}{O^ + }$ . According to IUPAC nomenclature hydronium ion is called oxonium ion. Hydronium ions are highly reactive and exist as solvated in water. It exists as a tri-hydrated hydronium ion with chemical formula is ${H_3}{O^ + } \cdot 3{H_2}O$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




