
Draw boundary surface diagrams of any four orbitals of subshell.
Answer
587.1k+ views
Hint: We can define a boundary surface diagram that has a good diagrammatical that represents shapes of atomic orbitals. We know there are four different kinds of orbitals, represented as s, p, d and f each with a different shape. Of the four, s and p orbitals are considered because these orbitals are of most common in organic and biological chemistry. We must remember that the s-orbital is spherical in shape with the nucleus that lies at its centre, p-orbitals are dumbbell-shaped and 4 of the five d orbitals have the shape of cloverleaf. The fifth d orbital looks like an elongated dumbbell with a doughnut about its middle. The orbitals in an atom are structured into various layers or electron shells.
Complete step by step answer: Now we are going to discuss about the features of a boundary surface diagram as,
Shape of the surface diagram: The boundary surface diagram of an orbital is independent of principle quantum number.
- Nodes in the surface diagram: The region that has very low probability density that goes to zero is called as nodes. Boundary surface diagram of s-orbital contains (n-1) nodes with ‘n’ principle quantum number. This type of node is observed in the surface diagram of p, d and f orbitals.
- Size of the surface diagram: The boundary surface diagram of an orbital raise in size or volume with raise in principle quantum number (n).
For any four orbitals of subshell we can give the boundary surface diagram as follows,
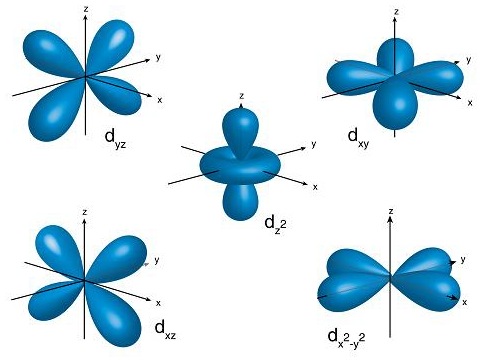
Note: We must remember that the s, p, d, and f are respectively sharp, primary, diffuse, and fundamental. We can give the magnetic orbital quantum number for d orbitals as (-2,-1, 0, 1, 2). Hence, we can say that there are five d-orbitals. These orbitals are designated as \[dxy\],\[dyz\], \[dxz\], \[{d_{{x^2}-{y^2}}}\] and \[d{z^2}\]. Out of these 5 d orbitals, shapes of the first 4 d-orbitals are similar to each other that are different from the \[d{z^2}\] orbital whereas the energy of all five d orbitals is the same.
Complete step by step answer: Now we are going to discuss about the features of a boundary surface diagram as,
Shape of the surface diagram: The boundary surface diagram of an orbital is independent of principle quantum number.
- Nodes in the surface diagram: The region that has very low probability density that goes to zero is called as nodes. Boundary surface diagram of s-orbital contains (n-1) nodes with ‘n’ principle quantum number. This type of node is observed in the surface diagram of p, d and f orbitals.
- Size of the surface diagram: The boundary surface diagram of an orbital raise in size or volume with raise in principle quantum number (n).
For any four orbitals of subshell we can give the boundary surface diagram as follows,

Note: We must remember that the s, p, d, and f are respectively sharp, primary, diffuse, and fundamental. We can give the magnetic orbital quantum number for d orbitals as (-2,-1, 0, 1, 2). Hence, we can say that there are five d-orbitals. These orbitals are designated as \[dxy\],\[dyz\], \[dxz\], \[{d_{{x^2}-{y^2}}}\] and \[d{z^2}\]. Out of these 5 d orbitals, shapes of the first 4 d-orbitals are similar to each other that are different from the \[d{z^2}\] orbital whereas the energy of all five d orbitals is the same.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




