
Draw structural formula for each of the following compounds:
Vinegar
Answer
564.6k+ views
Hint Structural formula identifies the location of chemical bonds between the atoms of a molecule. They are particularly useful for showing how compounds with the identical kind and number of atoms differ.
Complete answer:
- The structural formula or the molecular formula of an organic compound shows every bond between every atom within the molecule.
- A structural formula consists of symbols for the atoms connected by short lines that represent chemical bonds—one, two, or three lines standing for single, double, or triple bonds, respectively.
- The structural formula of vinegar is shown below
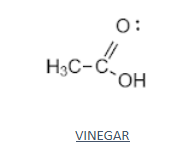
- Organic compounds, from any of the large classes of chemical compounds having one or more atoms of carbon are covalently linked to atoms of other elements, usually hydrogen, oxygen, or nitrogen.
-The few carbon-containing compounds not classified as organic include carbides, carbonates, and cyanides.
- Therefore, Vinegar is described as an organic compound, with the functional group carboxylic acid.
Additional information:
The main source of the carbon in organic compounds is carbon dioxide in the atmosphere. Plants use sunlight to convert carbon dioxide and water, which is an inorganic-compound, into sugar (an organic compound) through the process of photosynthesis.
Note: Carbon has four valence electrons, which means that each carbon atom can form a maximum of four bonds with other atoms. And due to the number of bonds that carbon can form with other atoms, organic compounds are often very complex.
Complete answer:
- The structural formula or the molecular formula of an organic compound shows every bond between every atom within the molecule.
- A structural formula consists of symbols for the atoms connected by short lines that represent chemical bonds—one, two, or three lines standing for single, double, or triple bonds, respectively.
- The structural formula of vinegar is shown below

- Organic compounds, from any of the large classes of chemical compounds having one or more atoms of carbon are covalently linked to atoms of other elements, usually hydrogen, oxygen, or nitrogen.
-The few carbon-containing compounds not classified as organic include carbides, carbonates, and cyanides.
- Therefore, Vinegar is described as an organic compound, with the functional group carboxylic acid.
Additional information:
The main source of the carbon in organic compounds is carbon dioxide in the atmosphere. Plants use sunlight to convert carbon dioxide and water, which is an inorganic-compound, into sugar (an organic compound) through the process of photosynthesis.
Note: Carbon has four valence electrons, which means that each carbon atom can form a maximum of four bonds with other atoms. And due to the number of bonds that carbon can form with other atoms, organic compounds are often very complex.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




