
Draw the chain isomers of hexane (${{C}_{6}}{{H}_{14}}$ ).
Answer
564.9k+ views
Hint: The isomers which have the same molecular formula and difference in their arrangement of carbon atoms in a chain are called chain isomers. Chain isomerism is possible in case of the compounds having more than two carbon atoms.
Complete Solution :
- In the question it is given that to draw the chain isomers of hexane.
- The molecular formula of hexane is ${{C}_{6}}{{H}_{14}}$ .
- Means there are six carbons and 14 hydrogens in hexane molecular formula.
- The possible structures of the chain isomers for hexane with the molecular formula ${{C}_{6}}{{H}_{14}}$ are as follows.
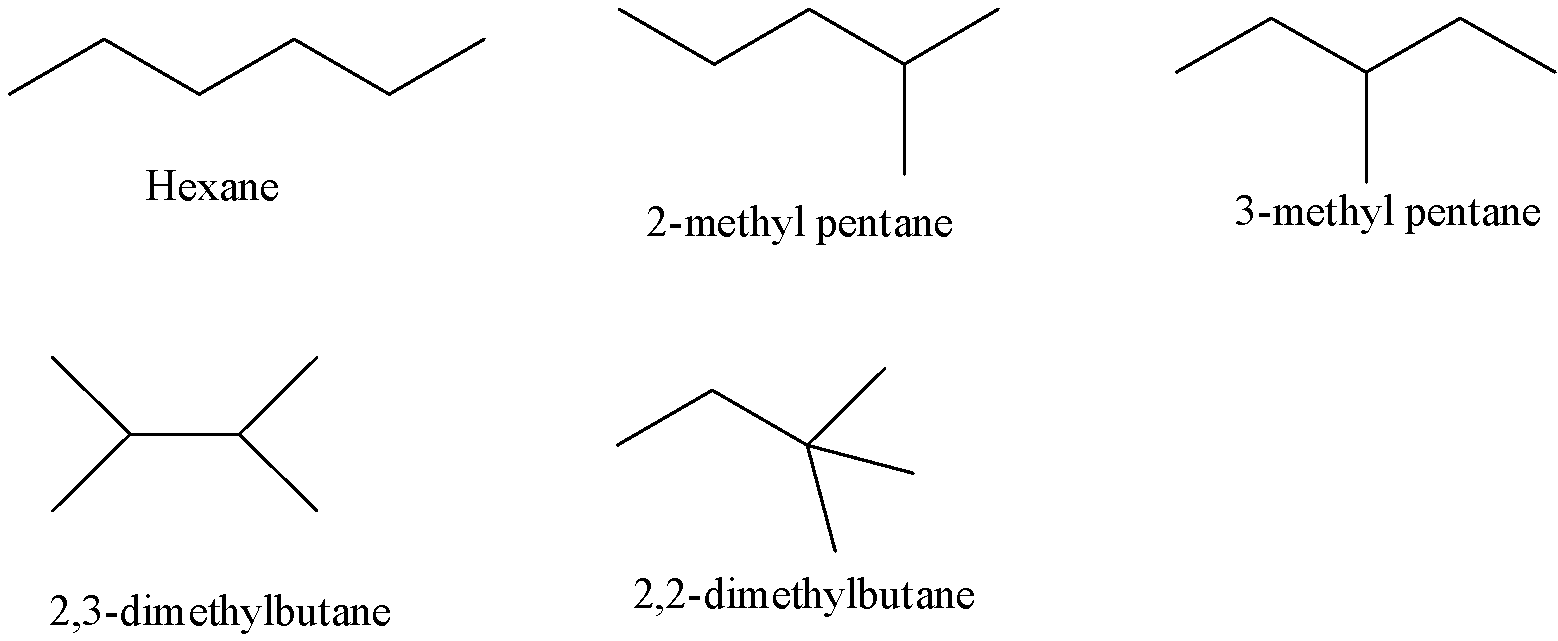
- From the above structures we can say that there are five isomers possible for hexane.
- The five isomers possible for hexane are n- hexane, 2- methyl pentane, 3- methyl pentane, 2, 3-dimethylbutane and 2, 2- dimethylbutane.
- 2- methyl pentane is also called Isohexane.
- 2, 2- dimethyl butane also called Neohexane.
- These five isomers are called constitutional isomers because each structure contains the same number of carbon atoms and the same number of hydrogen atoms.
- The above five structures have the same molecular formula but different in the arrangement of carbon atoms in the main chain.
Note: The number of possible arrangements of carbon atoms in the main chain produces the isomers of the compound. The chain isomers will have the same molecular formula but have differences in the arrangement of the carbon atoms with respect to chain.
Complete Solution :
- In the question it is given that to draw the chain isomers of hexane.
- The molecular formula of hexane is ${{C}_{6}}{{H}_{14}}$ .
- Means there are six carbons and 14 hydrogens in hexane molecular formula.
- The possible structures of the chain isomers for hexane with the molecular formula ${{C}_{6}}{{H}_{14}}$ are as follows.

- From the above structures we can say that there are five isomers possible for hexane.
- The five isomers possible for hexane are n- hexane, 2- methyl pentane, 3- methyl pentane, 2, 3-dimethylbutane and 2, 2- dimethylbutane.
- 2- methyl pentane is also called Isohexane.
- 2, 2- dimethyl butane also called Neohexane.
- These five isomers are called constitutional isomers because each structure contains the same number of carbon atoms and the same number of hydrogen atoms.
- The above five structures have the same molecular formula but different in the arrangement of carbon atoms in the main chain.
Note: The number of possible arrangements of carbon atoms in the main chain produces the isomers of the compound. The chain isomers will have the same molecular formula but have differences in the arrangement of the carbon atoms with respect to chain.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




