
Draw the cis and Trans structures of\[hex - 2 - ene\]. Which isomer will have higher boiling point and why?
Answer
561.3k+ views
Hint: To answer this question first we need to know about the structure of\[hex - 2 - ene\]. We also should know about how to draw cis and Trans structure of that compound (\[hex - 2 - ene\]).With regards to science, cis demonstrates that the useful gatherings are on a similar side of the carbon chain while trans passes on that useful gatherings are on opposite sides of the carbon chain. And we also know that boiling point is dependent on dipole dipole interaction. So, we need to know about how dipole dipole interaction works between the atoms of each molecule and how it is connected with boiling point.
Complete step by step answer:
The structure of \[hex - 2 - ene\] is:
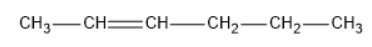
The two isomers obtained from \[hex - 2 - ene\] is below:
1) Cis-isomer:
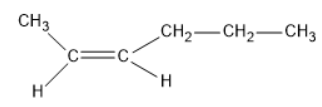
2) Trans-isomer:
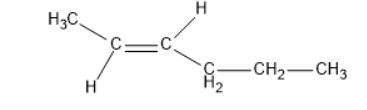
The dipole moment of cis-compound is an amount of the dipole moments of C-CH3 and C-CH2-CH2-CH3 bonds acting in a similar way. The dipole moment of trans-compound is the resultant of the dipole moments of C-CH3 and C-CH2-CH2-CH3 bonds acting inversely. Thus, cis-isomer is more polar than trans-isomer. The higher the extremity, the more noteworthy is that the inter atomic dipole-dipole connection, and therefore higher is going to be the limit.
Henceforth, the cis-isomer will have higher boiling point than the trans-isomer.
Note:Cis-trans isomers are stereoisomers, that is, sets of particles that have similar equations yet whose utilitarian gatherings are pivoted into an alternate direction in three-dimensional space. It isn't to be mistaken for E–Z isomerism, which is an absolute stereo chemical depiction. Stereoisomers contain double bonds that don't rotate, or they may contain ring structures, where the pivot of bonds is confined or prevented.
Complete step by step answer:
The structure of \[hex - 2 - ene\] is:
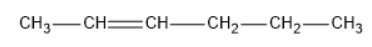
The two isomers obtained from \[hex - 2 - ene\] is below:
1) Cis-isomer:

2) Trans-isomer:

The dipole moment of cis-compound is an amount of the dipole moments of C-CH3 and C-CH2-CH2-CH3 bonds acting in a similar way. The dipole moment of trans-compound is the resultant of the dipole moments of C-CH3 and C-CH2-CH2-CH3 bonds acting inversely. Thus, cis-isomer is more polar than trans-isomer. The higher the extremity, the more noteworthy is that the inter atomic dipole-dipole connection, and therefore higher is going to be the limit.
Henceforth, the cis-isomer will have higher boiling point than the trans-isomer.
Note:Cis-trans isomers are stereoisomers, that is, sets of particles that have similar equations yet whose utilitarian gatherings are pivoted into an alternate direction in three-dimensional space. It isn't to be mistaken for E–Z isomerism, which is an absolute stereo chemical depiction. Stereoisomers contain double bonds that don't rotate, or they may contain ring structures, where the pivot of bonds is confined or prevented.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




