
Draw the electron dot structure of ammonia molecules.
A.
B.\[HCl\]
C.\[NaCl\]
D.None of these

Answer
500.4k+ views
Hint: Ammonia is a molecule formed from the combining of nitrogen and hydrogen atoms. There are three hydrogen atoms and one nitrogen atom. The valence electrons are only involved in the bond formation. Nitrogen has five valence electrons and hydrogen has only one valence electron.
Complete answer:
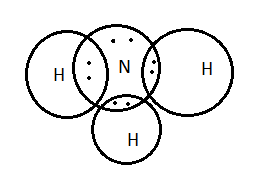
Hydrogen is the element with atomic number \[1\] and nitrogen is the element with atomic number \[7\] . Hydrogen has only one valence electron and nitrogen has five valence electrons. Thus, the total valence electrons involved in the nitrogen molecule are \[8\] as the three hydrogen atoms have \[3\] valence electrons and nitrogen have \[5\] valence electrons.
Ammonia is the molecule with pyramidal geometry; due to the presence of lone pairs of electrons on nitrogen it is a base.
Lewis dot structure or diagram gives information about the valence electrons and bonds. The atoms are represented in the dark circles. The electrons were represented as black dots or cross marks.
In the given options, option A has four circles which are the representation of four atoms and the eight valence electrons are represented in between the two atoms.
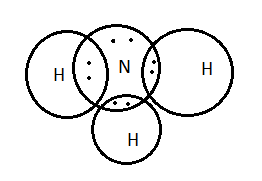
Thus, option A is the correct one.
Note:
Lewis dot structure is also known as electron dot structure. It helps to find out the valence electrons within the molecule. The electrons which were involved in the bond and those which were not (lone pair of electrons) involved can be predicted from the Lewis dot diagram.
Complete answer:
Hydrogen is the element with atomic number \[1\] and nitrogen is the element with atomic number \[7\] . Hydrogen has only one valence electron and nitrogen has five valence electrons. Thus, the total valence electrons involved in the nitrogen molecule are \[8\] as the three hydrogen atoms have \[3\] valence electrons and nitrogen have \[5\] valence electrons.
Ammonia is the molecule with pyramidal geometry; due to the presence of lone pairs of electrons on nitrogen it is a base.
Lewis dot structure or diagram gives information about the valence electrons and bonds. The atoms are represented in the dark circles. The electrons were represented as black dots or cross marks.
In the given options, option A has four circles which are the representation of four atoms and the eight valence electrons are represented in between the two atoms.

Thus, option A is the correct one.
Note:
Lewis dot structure is also known as electron dot structure. It helps to find out the valence electrons within the molecule. The electrons which were involved in the bond and those which were not (lone pair of electrons) involved can be predicted from the Lewis dot diagram.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




