
Draw the electron dot structure of ethane molecule $\left( {{C}_{2}}{{H}_{6}} \right)$
Answer
570k+ views
Hint: The electronic dot structure is the Lewis electron structure or Lewis dot structure, which gives the valency of each atom. We should know that there are two carbon atoms which are connected to six hydrogen atoms in ethane.
Complete answer:
So in the question, it is asked to draw the electron dot structure of an ethane molecule. Electronic dot structure is the Lewis dot structure or Lewis electron dot structure in which we draw the structure of the molecule by satisfying octet configuration or stable noble gas configuration for each atom.
We know that ethane is a compound in the homologous series of alkanes having the general formulae, ${{C}_{n}}{{H}_{2n+2}}$, where n is the number of carbon atoms present in the alkane.
Now we should know the number of electrons in the valence shell of the atom, as we draw the structure combining the valence shell electrons.
The electronic configuration of H is, $1{{s}^{1}}$ since it has only one electron and has the atomic number 1.
So the valency of hydrogen is +1.
The electronic configuration of C is, $1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}$, as its atomic number is six and has four electrons in the valence shell. So its valency is 4.
Now let’s see the electronic dot structure of ethane,
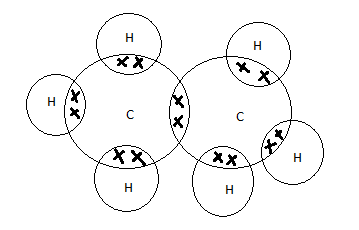
So we have drawn the picture satisfying all the valencies of all the atoms and now the atoms have the stable configuration.
With the help of Lewis dot structure we could say the number of bonds an atom makes and the type of bond it forms.
Here the carbon and hydrogen present are forming only the covalent bond, through the sharing of electrons and the structure of ethane is,
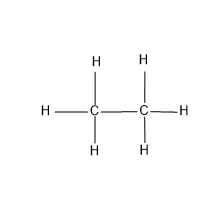
Note: The ethane is the second compound in the homologous series of alkanes as it contains two carbon, the first compound with one carbon is methane, with three carbon is propane, with four carbon butane etc. We should always give attention to the valence electrons and should always satisfy the valency and the octet configuration of the atoms in the compound.
Complete answer:
So in the question, it is asked to draw the electron dot structure of an ethane molecule. Electronic dot structure is the Lewis dot structure or Lewis electron dot structure in which we draw the structure of the molecule by satisfying octet configuration or stable noble gas configuration for each atom.
We know that ethane is a compound in the homologous series of alkanes having the general formulae, ${{C}_{n}}{{H}_{2n+2}}$, where n is the number of carbon atoms present in the alkane.
Now we should know the number of electrons in the valence shell of the atom, as we draw the structure combining the valence shell electrons.
The electronic configuration of H is, $1{{s}^{1}}$ since it has only one electron and has the atomic number 1.
So the valency of hydrogen is +1.
The electronic configuration of C is, $1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}$, as its atomic number is six and has four electrons in the valence shell. So its valency is 4.
Now let’s see the electronic dot structure of ethane,

So we have drawn the picture satisfying all the valencies of all the atoms and now the atoms have the stable configuration.
With the help of Lewis dot structure we could say the number of bonds an atom makes and the type of bond it forms.
Here the carbon and hydrogen present are forming only the covalent bond, through the sharing of electrons and the structure of ethane is,

Note: The ethane is the second compound in the homologous series of alkanes as it contains two carbon, the first compound with one carbon is methane, with three carbon is propane, with four carbon butane etc. We should always give attention to the valence electrons and should always satisfy the valency and the octet configuration of the atoms in the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




