
Draw the electronic dot structure of propanal.
Answer
561k+ views
Hint: First of all, we need to know what the electron dot structure means. The electron dot structure of an atom is the structure in which dots are used to represent the number of valence electrons in that atom. This method was introduced by Gilbert Newton Lewis in 1916 and hence this method of representation of valence electrons is also called Lewis dot structure.
Complete answer:
Propanal is an organic aldehydic compound with molecular formula as \[CH_3CH_2CHO\]. It is liquid at room temperature and generally gives a fruity smell. It is a widely used compound in the laboratory and large scale industries.
In the electron dot structure of any compound, firstly we write the symbol of all the atoms in that compound, for example, we write C for carbon atoms.
Then, we assign a few dots or cross around each atom which represents their valency. Thus, the number of dots is equal to the valency of that atom, for example, we surround a carbon atom by 4 dots or cross because the valency of carbon is 4.
The electronic dot structure of propanal is given below:
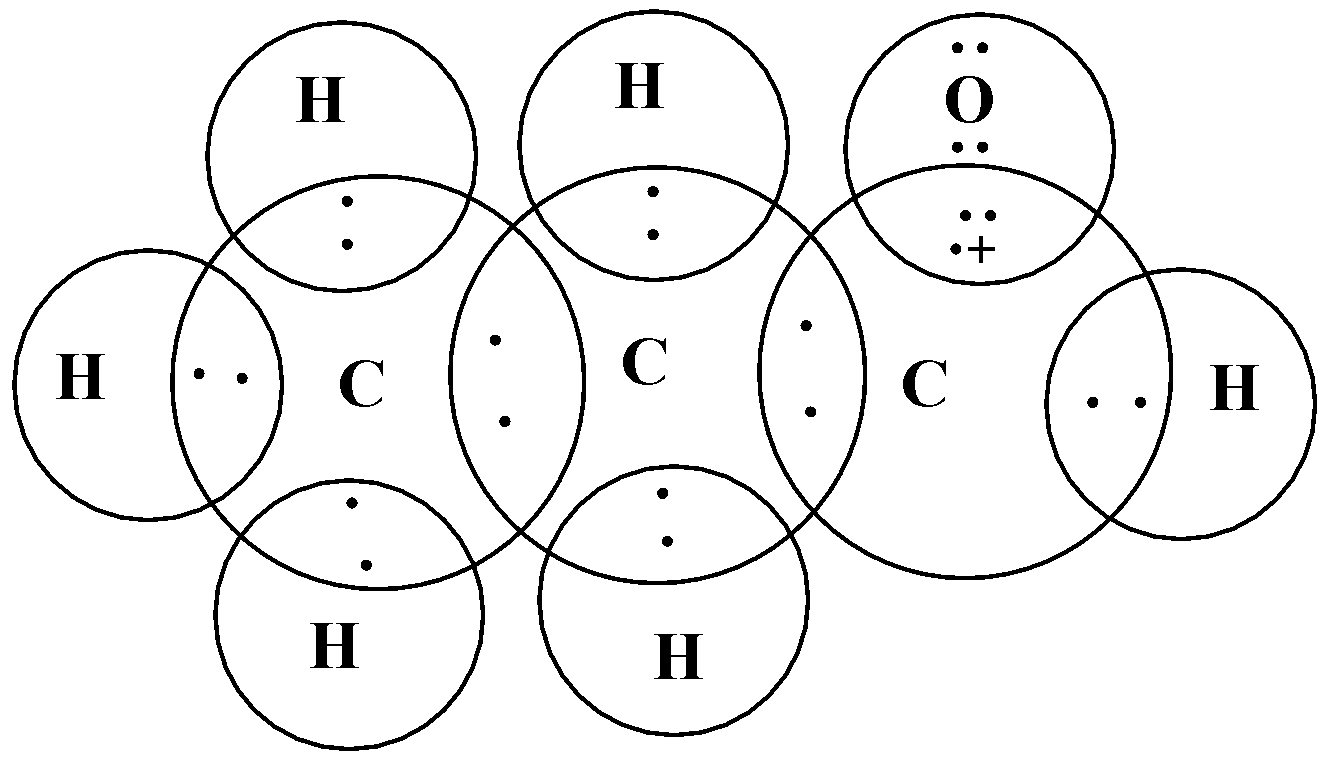
In this diagram, we assigned cross to represent valence electrons of carbon and dots to represent valence electrons of H and O atoms.
At last, we drew circles to show and confirm the complete octet (in case of carbon and oxygen atom). The hydrogen atom can only acquire 2 electrons to complete its valence thus the circle for a hydrogen atom includes only 2 electrons.
Note: Propanal is used in the laboratory and commercially to produce several other bigger molecules. It is used in the production of trimethylolethane .
Complete answer:
Propanal is an organic aldehydic compound with molecular formula as \[CH_3CH_2CHO\]. It is liquid at room temperature and generally gives a fruity smell. It is a widely used compound in the laboratory and large scale industries.
In the electron dot structure of any compound, firstly we write the symbol of all the atoms in that compound, for example, we write C for carbon atoms.
Then, we assign a few dots or cross around each atom which represents their valency. Thus, the number of dots is equal to the valency of that atom, for example, we surround a carbon atom by 4 dots or cross because the valency of carbon is 4.
The electronic dot structure of propanal is given below:

In this diagram, we assigned cross to represent valence electrons of carbon and dots to represent valence electrons of H and O atoms.
At last, we drew circles to show and confirm the complete octet (in case of carbon and oxygen atom). The hydrogen atom can only acquire 2 electrons to complete its valence thus the circle for a hydrogen atom includes only 2 electrons.
Note: Propanal is used in the laboratory and commercially to produce several other bigger molecules. It is used in the production of trimethylolethane .
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




