
Draw the flowchart for obtaining different gases from air.
Answer
588.6k+ views
Hint: The main components of air are nitrogen, oxygen and argon. These gases cannot be separated easily in their gaseous form. So, we need to change their state into liquid. The method of separation is fractional distillation. Try to find the values of their boiling point temperatures to understand how the process happens and which gas is obtained first.
Complete step-by-step answer:
Distillation is the process of separating the components or substances present in a liquid mixture through selective boiling and condensation. Distillation may result in complete or partial separation based on the difference in boiling points of the substances present in the liquid mixture.
In either case, the process of distillation uses the concept of relative volatility of the components of the mixture. It has many uses like:
- Separation of fermented products to produce high alcohol content beverages
- Desalination
- Separation of air into its components for industrial use (cryogenic distillation)
- Refining of crude oil
- Oil stabilisation in petroleum industry
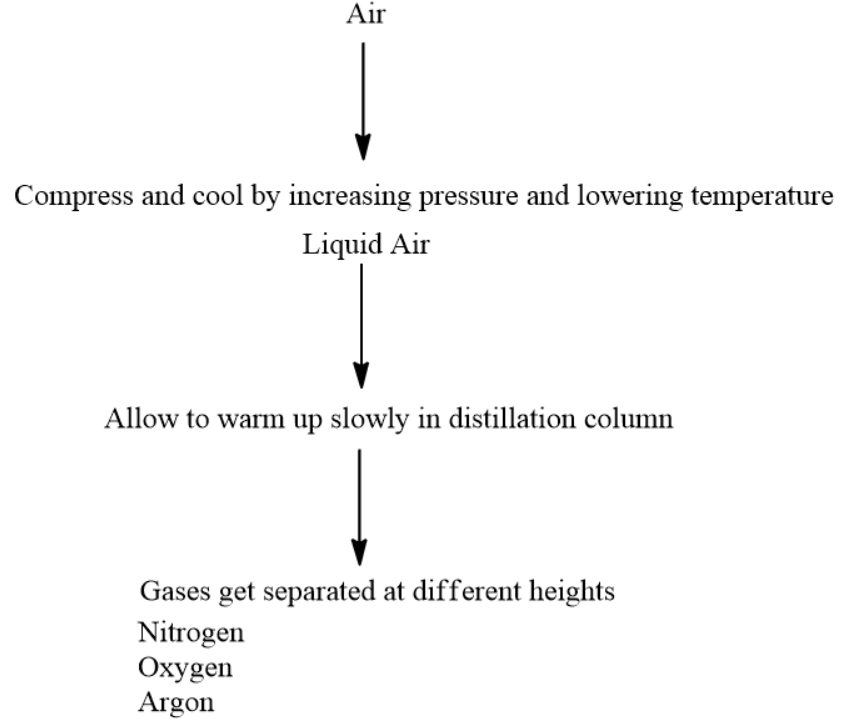
In the above case, the gases cannot be separated easily. This is the reason why the gases are first liquified. Once they are compressed to a liquid state with high pressure and low temperature, we slowly start heating them.
As we increase the temperature, the gases start to rise. This is the crucial stage of distillation. We will start collecting specific gases at their boiling point temperatures.
The main components of air are :
- Nitrogen (78.1%)
- Oxygen (20.9%)
- Argon (0.9%)
The flow chart for obtaining different gases from air is given below:
Note: When the difference between the boiling temperatures of the components of liquid mixture is less, we use the process of fractional distillation. Fractional distillation is the separation of a liquid mixture into its component parts, or fractions. Chemical compounds are separated by heating to a temperature at which more than one fractions of the mixture will vaporise.
Complete step-by-step answer:
Distillation is the process of separating the components or substances present in a liquid mixture through selective boiling and condensation. Distillation may result in complete or partial separation based on the difference in boiling points of the substances present in the liquid mixture.
In either case, the process of distillation uses the concept of relative volatility of the components of the mixture. It has many uses like:
- Separation of fermented products to produce high alcohol content beverages
- Desalination
- Separation of air into its components for industrial use (cryogenic distillation)
- Refining of crude oil
- Oil stabilisation in petroleum industry
In the above case, the gases cannot be separated easily. This is the reason why the gases are first liquified. Once they are compressed to a liquid state with high pressure and low temperature, we slowly start heating them.
As we increase the temperature, the gases start to rise. This is the crucial stage of distillation. We will start collecting specific gases at their boiling point temperatures.
The main components of air are :
- Nitrogen (78.1%)
- Oxygen (20.9%)
- Argon (0.9%)
The flow chart for obtaining different gases from air is given below:

Note: When the difference between the boiling temperatures of the components of liquid mixture is less, we use the process of fractional distillation. Fractional distillation is the separation of a liquid mixture into its component parts, or fractions. Chemical compounds are separated by heating to a temperature at which more than one fractions of the mixture will vaporise.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




