
How can I draw the following amines: butan-1-amine, pentan-2-amine, propan-1,2-diamine?
Answer
533.4k+ views
Hint: Amines are the functional groups that contain $N{{H}_{2}}$, or NH species. Amines are classified as primary, secondary and tertiary amines. The structure of any organic compound tells us the position of functional groups, and arrangement of carbon chains.
Complete answer: Amines are nitrogen containing compounds. The drawing of amines can be done with the recognition of the hydrocarbon chain and then by adding the amines. The given compounds will be drawn as:
Butan-1-amine, this compound contains a butane carbon chain, which is a 4 carbon chain, and an amine group is placed at first carbon. The formula will be ${{H}_{2}}NC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}$. Through this formula the structure can be drawn as,
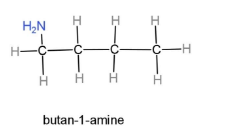
Pentan-2-amine, this compound contains a pentane carbon chain, which is a 5 carbon chain, and an amine group is placed at second carbon. The formula will be $C{{H}_{3}}CH(N{{H}_{2}})C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}$. Through this formula the structure can be drawn as,
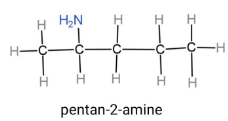
Propan-1,2-diamine, this compound contains a propane carbon chain, which is a 53carbon chain, and 2 amine groups are placed at first and second carbon. The formula will be ${{H}_{2}}NC{{H}_{2}}CH(N{{H}_{2}})C{{H}_{3}}$. Through this formula the structure can be drawn as,
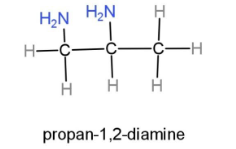
Hence, the structures of butan-1-amine, pentan-2-amine, propan-1,2-diamine can be drawn.
Note: Amines attached only with 1 carbon are called primary amines. Those attached with 2 carbons are called secondary amines, while those amines attached with 3 carbon atoms are called tertiary amines. We can see that all the amines are primary in the three compounds stated in the problem.
Complete answer: Amines are nitrogen containing compounds. The drawing of amines can be done with the recognition of the hydrocarbon chain and then by adding the amines. The given compounds will be drawn as:
Butan-1-amine, this compound contains a butane carbon chain, which is a 4 carbon chain, and an amine group is placed at first carbon. The formula will be ${{H}_{2}}NC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}$. Through this formula the structure can be drawn as,

Pentan-2-amine, this compound contains a pentane carbon chain, which is a 5 carbon chain, and an amine group is placed at second carbon. The formula will be $C{{H}_{3}}CH(N{{H}_{2}})C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}$. Through this formula the structure can be drawn as,

Propan-1,2-diamine, this compound contains a propane carbon chain, which is a 53carbon chain, and 2 amine groups are placed at first and second carbon. The formula will be ${{H}_{2}}NC{{H}_{2}}CH(N{{H}_{2}})C{{H}_{3}}$. Through this formula the structure can be drawn as,

Hence, the structures of butan-1-amine, pentan-2-amine, propan-1,2-diamine can be drawn.
Note: Amines attached only with 1 carbon are called primary amines. Those attached with 2 carbons are called secondary amines, while those amines attached with 3 carbon atoms are called tertiary amines. We can see that all the amines are primary in the three compounds stated in the problem.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




