
How would you draw the four possible stereoisomers of $3 - bromo - 4 - fluorohexane$
Answer
546.3k+ views
Hint: Start by fischer projection which is the easiest representation of stereoisomers thus assign the vertical chain of carbon as largest number and then try to figure out different positions for fluorine. When you will assign the position to fluorine the alternated ${3^{rd}}$ position is to be occupied by another atom to bromine.
Complete step-by-step answer:
Here let’s start by giving an idea that we are talking about stereoisomers, so the difference of positions of atoms is given by the fischer structure. Our first step is to draw a basic skeleton which you see here in the diagram below. So we have taken six carbon atoms on the vertical line while we leave four positions horizontally. These horizontal positions have one fluorine, one bromine and two hydrogens.
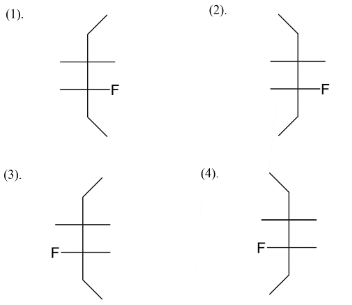
We add fluorine at the $C - 4$ position like in four ways, we get an idea about the four stereoisomers that we will get after assigning positions to bromine also. Next step is to assign bromine as position $C - 3$ alternating right-left-right left and vice versa. So at last we left with fulfilling valences of carbon. So, four stereoisomers of $3 - bromo - 4 - fluorohexane$ are given above.
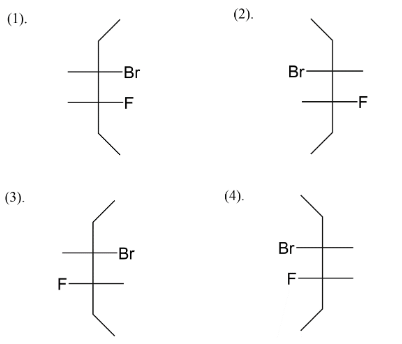
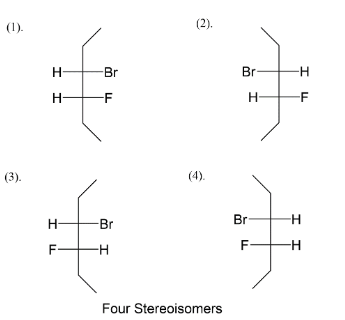
Note: We know there are four types of representation for stereoisomers which are flying wedge, fisher, sawhorse and Newman. Among the four types we usually see fisher structures when it comes to stereoisomers. You have seen the fisher structure in carbohydrates where it was initially thought that all carbohydrates possess fisher structure and gave reaction by it.
Complete step-by-step answer:
Here let’s start by giving an idea that we are talking about stereoisomers, so the difference of positions of atoms is given by the fischer structure. Our first step is to draw a basic skeleton which you see here in the diagram below. So we have taken six carbon atoms on the vertical line while we leave four positions horizontally. These horizontal positions have one fluorine, one bromine and two hydrogens.

We add fluorine at the $C - 4$ position like in four ways, we get an idea about the four stereoisomers that we will get after assigning positions to bromine also. Next step is to assign bromine as position $C - 3$ alternating right-left-right left and vice versa. So at last we left with fulfilling valences of carbon. So, four stereoisomers of $3 - bromo - 4 - fluorohexane$ are given above.


Note: We know there are four types of representation for stereoisomers which are flying wedge, fisher, sawhorse and Newman. Among the four types we usually see fisher structures when it comes to stereoisomers. You have seen the fisher structure in carbohydrates where it was initially thought that all carbohydrates possess fisher structure and gave reaction by it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




