
Draw the Lewis dot structure of the following molecules and ions: ${{H}_{2}}S,\text{ SiC}{{\text{l}}_{4}},\text{ Be}{{\text{F}}_{2}},\text{ C}{{\text{O}}_{3}}^{2-}\text{ and HCOOH}$
Answer
582.3k+ views
HINT: We can draw electron dot structure easily by calculating the valence electrons on each atom present in the given molecules. While drawing the dot structures here remember that the number of valence electrons in silicon is 4 and that for sulphur, beryllium, fluorine, carbon and oxygen is 6, 2, 7, 4 and 6 respectively. Use this to draw the structures.
COMPLETE STEP BY STEP SOLUTION: We also know Lewis dot structures as electron dot structure. They are diagrams that show the bonding between atoms or molecules and the lone pairs of electrons existing in the molecule.
To draw the structure, we need to follow some steps. They are-
The total number of electrons represented in the electron dot structure is the sum of the total number of valence electrons present in each atom of the molecule.
Electrons other than the valence electrons are not shown in a valence dot structure.
After we have determined the number of valence electrons, we can place them in the dot structure following these steps-
-If we are drawing it for a molecule which consists of two or more atoms, the atoms are connected by single bonds.
-Electrons should be placed as lone pairs. One pair of dots for each pair of electrons should be placed.
-Lone pairs should be placed on outer atoms until each atom has eight electrons. If there are any extra electrons left, they should be placed on the central atom.
-When all the lone pairs are placed, if the central atom did not complete its octet, a double bond should be placed.
Now we can draw the Lewis dot structure of the given structures following the above rules as -
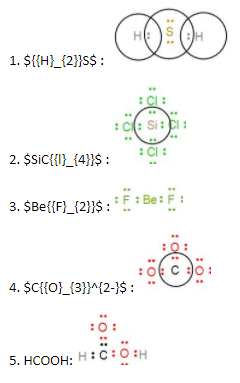
From the above diagrams we can see the required Lewis dot structures of the given molecules.
NOTE: To draw electron dot structure, it is important that we remember the structure of compounds if the formula is not given. It is also important to remember the atomic numbers in order to find out the number of valence electrons.
COMPLETE STEP BY STEP SOLUTION: We also know Lewis dot structures as electron dot structure. They are diagrams that show the bonding between atoms or molecules and the lone pairs of electrons existing in the molecule.
To draw the structure, we need to follow some steps. They are-
The total number of electrons represented in the electron dot structure is the sum of the total number of valence electrons present in each atom of the molecule.
Electrons other than the valence electrons are not shown in a valence dot structure.
After we have determined the number of valence electrons, we can place them in the dot structure following these steps-
-If we are drawing it for a molecule which consists of two or more atoms, the atoms are connected by single bonds.
-Electrons should be placed as lone pairs. One pair of dots for each pair of electrons should be placed.
-Lone pairs should be placed on outer atoms until each atom has eight electrons. If there are any extra electrons left, they should be placed on the central atom.
-When all the lone pairs are placed, if the central atom did not complete its octet, a double bond should be placed.
Now we can draw the Lewis dot structure of the given structures following the above rules as -

From the above diagrams we can see the required Lewis dot structures of the given molecules.
NOTE: To draw electron dot structure, it is important that we remember the structure of compounds if the formula is not given. It is also important to remember the atomic numbers in order to find out the number of valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




