
Draw the Lewis structure for carbonate ion, ${\text{CO}}_{\text{3}}^{{\text{2 - }}}$.
Answer
584.1k+ views
Hint: Carbonate ion is a polyatomic ion. It is the simplest oxocarbon anion consisting of one carbon atom and two oxygen atoms. The ion has a trigonal planar arrangement, with a molecular mass of 60 u. Carbonate ion carries a formal charge of -2. It is a conjugate base of bicarbonate ion (${\text{HCO}}_{\text{3}}^{\text{ - }}$) which in turn a conjugate base of carbonic acid (${{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}$).
Complete step by step answer:
Now we will be looking into the Lewis structure of carbonate ion in a more detailed manner:
The Lewis structure of the anion has one double bond between a neutral oxygen atom and carbon. And two single bonds between carbon and two negatively charged oxygen atoms.
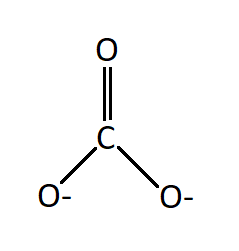
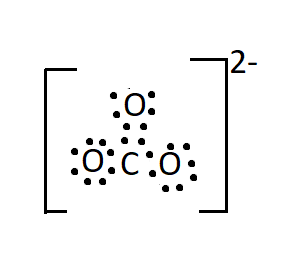
Seeing the structure one would imagine that in carbonate ion two bonds will be the same in length (the two single bonds) and will be longer as compared to the third bond which is a double bond. But it has been found that all the three bonds are of the same length and this observation is attributed to the resonance effect.
There are three resonating structures of carbonate ions and a double bond is interchangeable thus giving each bond an identical bond length.
Note:
We have discussed the Lewis structure of the carbonate ion in the same context and it is important to know what Lewis structure is.
-Lewis structure is sometimes also called Lewis dot diagrams, Lewis dot formulas, Lewis dot structure, Lewis electron dot structure or electron dot structure.
-The diagram shows the bonding between atoms and the lone pair of electrons in a molecule in which electrons are represented as a dot.
Complete step by step answer:
Now we will be looking into the Lewis structure of carbonate ion in a more detailed manner:
The Lewis structure of the anion has one double bond between a neutral oxygen atom and carbon. And two single bonds between carbon and two negatively charged oxygen atoms.


Seeing the structure one would imagine that in carbonate ion two bonds will be the same in length (the two single bonds) and will be longer as compared to the third bond which is a double bond. But it has been found that all the three bonds are of the same length and this observation is attributed to the resonance effect.
There are three resonating structures of carbonate ions and a double bond is interchangeable thus giving each bond an identical bond length.
Note:
We have discussed the Lewis structure of the carbonate ion in the same context and it is important to know what Lewis structure is.
-Lewis structure is sometimes also called Lewis dot diagrams, Lewis dot formulas, Lewis dot structure, Lewis electron dot structure or electron dot structure.
-The diagram shows the bonding between atoms and the lone pair of electrons in a molecule in which electrons are represented as a dot.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




