
How do you draw the Lewis structure for \[\;OPB{R_3}\]?
Answer
554.1k+ views
Hint: \[Of{\text{ }}phosphoryl{\text{ }}bromide\] There are \[6 + 5 + 3 \times 7 = 32\] electrons to allocate.
Lewis structures of \[O = PB{r_3}\] or \[^ - O{ - ^ + }PB{r_3}\] are suitable. The second one is most likely more normal at this point. Each structure appropriates \[16\] pairs of valence electrons as is required.
Complete step by step answer:
Lewis structure is fundamentally a realistic portrayal of the electron circulation around a particle. The significant motivation behind why learning Lewis dot structure is significant is that it helps in anticipating the number and kind of bonds which can be conformed to an atom. It additionally helps in anticipating the math of the atom. Figuring out how to make appropriate Lewis dot structures can help in taking care of the issue that a large portion of the understudies have.
Drawing the Lewis structure is significant as it is then just when one can compute the proper charge effectively.
Right off the bat, we need to Determine the quantity of valence electrons present in the particle.
\[
Oxygen \to 6 \\
Phosphorus \to 5 \\
Bromide \to 3 \times 7{\text{ }} = {\text{ }}21 \\
\]
Complete valence electron \[ = 6 + 5 + 21 = 32\]
At that point, attract a skeleton particle which the focal molecule interfaces with all the atoms utilizing a solitary bond \[P\] is a focus particle. Twofold bonds oxygen to \[P\] and puts \[4\] electrons on its external shell. At that point have a customary bond interfacing the three \[Br\] to the \[P\] and give them \[6\] electrons outwardly.
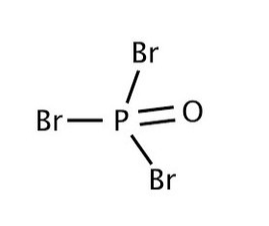
Lewis structures are truly useful with regards to finding out about the oxidation states, valence and the kind of holding. Be that as it may, actually, there are numerous exemptions with regards to the structure as a general rule. Atoms when all is said in done, attempt and look to half-fill or completely fill their valence electron shell. Be that as it may, the particles can do this and furthermore structure atoms which are not steady. Now and again, the focal atom can likewise frame more than different atoms associated with it. There are chances that the quantity of valence electrons can surpass in excess of 8 electrons. This can generally be seen in higher nuclear numbers.
Note:
Lewis structures are by and large supportive with regards to lighter elements and not that accommodating with regards to change metals including the two actinides and lanthanides. They portray the unpredictable plan of electrons around the particle.
Lewis structures of \[O = PB{r_3}\] or \[^ - O{ - ^ + }PB{r_3}\] are suitable. The second one is most likely more normal at this point. Each structure appropriates \[16\] pairs of valence electrons as is required.
Complete step by step answer:
Lewis structure is fundamentally a realistic portrayal of the electron circulation around a particle. The significant motivation behind why learning Lewis dot structure is significant is that it helps in anticipating the number and kind of bonds which can be conformed to an atom. It additionally helps in anticipating the math of the atom. Figuring out how to make appropriate Lewis dot structures can help in taking care of the issue that a large portion of the understudies have.
Drawing the Lewis structure is significant as it is then just when one can compute the proper charge effectively.
Right off the bat, we need to Determine the quantity of valence electrons present in the particle.
\[
Oxygen \to 6 \\
Phosphorus \to 5 \\
Bromide \to 3 \times 7{\text{ }} = {\text{ }}21 \\
\]
Complete valence electron \[ = 6 + 5 + 21 = 32\]
At that point, attract a skeleton particle which the focal molecule interfaces with all the atoms utilizing a solitary bond \[P\] is a focus particle. Twofold bonds oxygen to \[P\] and puts \[4\] electrons on its external shell. At that point have a customary bond interfacing the three \[Br\] to the \[P\] and give them \[6\] electrons outwardly.

Lewis structures are truly useful with regards to finding out about the oxidation states, valence and the kind of holding. Be that as it may, actually, there are numerous exemptions with regards to the structure as a general rule. Atoms when all is said in done, attempt and look to half-fill or completely fill their valence electron shell. Be that as it may, the particles can do this and furthermore structure atoms which are not steady. Now and again, the focal atom can likewise frame more than different atoms associated with it. There are chances that the quantity of valence electrons can surpass in excess of 8 electrons. This can generally be seen in higher nuclear numbers.
Note:
Lewis structures are by and large supportive with regards to lighter elements and not that accommodating with regards to change metals including the two actinides and lanthanides. They portray the unpredictable plan of electrons around the particle.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




